Psicose
 | |
| Names | |
|---|---|
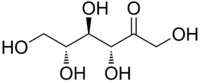
| IUPAC name
(3R,4R,5R)-1,3,4,5,6-Pentahydroxyhexan-2-one | |
| Other names
D-Psicose | |
| Identifiers | |
| 23140-52-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27605 |
| ChemSpider | 81254 |
| MeSH | psicose |
| PubChem | 90008 |
| |
| |
| Properties | |
| C6H12O6 | |
| Molar mass | 180.16 g·mol−1 |
| Melting point | 58 °C (136 °F; 331 K) [1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
D-Psicose (D-allulose, D-ribo-2-hexulose, C6H12O6) is a low-energy monosaccharide sugar present in small quantities in natural products. First identified in wheat more than 70 years ago, psicose is a C-3 epimer of D-fructose, and is present in small quantities in agricultural products and commercially prepared carbohydrate complexes. The sweetness of psicose is 70% of the sweetness of sucrose,[2] and health benefits of psicose may include improved insulin resistance, antioxidant enhancement and formation, and hypoglycemic controls.[3]
History
The first mass-production method for psicose was established when Ken Izumori at Kagawa University in Japan discovered the key enzyme, D-tagatose 3-epimerase, to convert fructose to D-psicose in 1994.[4][5] This method of production has a high yield, but suffers from a very high production cost.
CJ CheilJedang was the first company to file for GRAS status in the US.[6]
Commercial application of D-psicose
Commercial manufacturers and food laboratories are looking into properties of D-psicose that may differentiate it from sucrose and fructose sweeteners, including an ability to induce the high foaming property of egg white protein and the production of antioxidant substances produced through the Maillard reaction.[7]
Commercial uses of the sugar are underway by at least two manufacturers, both of whom are marketing their variants as low-calorie sweeteners in beverages, yogurt, ice cream, baked goods, and other typically high-calorie items. London-based Tate & Lyle released its proprietary variant of psicose, known as Dolcia Prima allulose,[8] and U.S.-based Anderson Global Group in conjunction with South Korean manufacturer CJ CheilJedang released its own proprietary variant into the North American market in 2015.[9][10]
The U.S. Food and Drug Administration (FDA) lists psicose as a generally recognized as safe (GRAS) product and has approved its use in a wide variety of products.[11] Psicose is not yet approved for use in the European Union.
A study sponsored by psicose producer Tate & Lyle showed that because psicose is not generally metabolized, its caloric value is significantly lower than table sugar - nearly zero.[2] The company maintains that psicose can benefit consumers who monitor their sugar intake because it does not impact the glycemic response significantly. A study cited by Tate & Lyle showed that when 25 g of psicose were ingested compared to 25 g of sucrose, psicose did not raise blood sugar levels above the baseline for two hours after ingestion.[12]
References
- ↑ Lide, David R.; Milne, G.W.A., eds. (30 Dec 1993). CRC Handbook of Data on Organic Compounds (3rd ed.). CRC Press. p. 4596.
- 1 2 Chung, Min-Yu; Oh, Deok-Kun; Lee, Ki Won (1 Feb 2012). "Hypoglycemic health benefits of D-psicose". J Agric Food Chem. ACS. 60 (4): 863–869. doi:10.1021/jf204050w. PMID 22224918.
- ↑ O’Donnell, Claudia (26 March 2014). "Formulating for a Sweet Perception With Natural Sweeteners". Food Processing. Retrieved 12 July 2015.
- ↑ Itoh, Hiromichi; Okaya, Hiroaki; Khan, Anisur Rahman; et al. (1994). "Purification and characterization of D-tagatose 3-epimerase from Pseudomonas sp. ST-24". Biosci Biotechnol Biochem. 58: 2168–2171. doi:10.1271/bbb.58.2168.
- ↑ Itoh, Hiromichi; Sato, Tomoko; Izumori, Ken (1995). "Preparation of D-psicose from D-fructose by immobilized D-tagatose 3-epimerase". J Fermentation and Bioengineering. Elsevier B.V. 80 (1): 101–103. doi:10.1016/0922-338X(95)98186-O.
- ↑ "GRAS Notice" (PDF). fda.gov. FDA. Retrieved 12 July 2015.
- ↑ Watson, Elaine (25 Feb 2015). "Tate & Lyle unveils Dolcia Prima allulose low-calorie-sugar: 'We believe this will change the food and beverage landscape forever'". foodnavigator-usa.com. William Reed Business Media SAS.
- ↑ Gelski, Jeff (30 June 2015). "New low-calorie sweetener to launch at I.F.T.". Food Business News. Retrieved 12 July 2015.
- ↑ "AllSweet". Anderson Global Group.
- ↑ "U.S. FDA. Agency Response Letter GRAS Notice No. GRN 000400.". FDA. Retrieved 12 July 2015.
- ↑ Kendall C, Wolever T, Jenkins A et al. "A Randomized, Controlled, Crossover Study to Assess the Effects of a Sweetener on Postprandial Glucose and Insulin Excursions in Healthy Subjects". May 2014. Glycemic Index Laboratories. Toronto, ON, Canada.