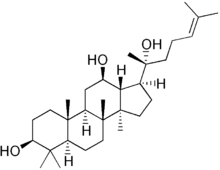
Protopanaxadiol
 | |
| Names | |
|---|---|
| IUPAC name
(3S,5R,8R,9R,10R,12R,13R,14R,17S)-17-[(2S)-2-hydroxy-6-methylhept-5-en-2-yl]-4,4,8,10,14-pentamethyl-2,3,5,6,7,9,11,12,13,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthrene-3,12-diol | |
| Other names
Dammar-24-ene-3β,12β,20-triol | |
| Identifiers | |
| 7755-01-3 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | PPD |
| ChEBI | CHEBI:76237 |
| ChEMBL | ChEMBL375563 |
| ChemSpider | 8095918 |
| ECHA InfoCard | 100.208.650 |
| PubChem | 9920281 |
| UNII | P6717R7BP8 |
| |
| |
| Properties | |
| C30H52O3 | |
| Molar mass | 460.74 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Protopanaxadiol (PPD) is an organic compound characterizing a group of ginsenosides. It is a dammarane-type tetracyclic terpene sapogenin found in ginseng (Panax ginseng) and in notoginseng (Panax pseudoginseng).[1][2]
Just what protopanaxadiol's metabolites do inside the human body is still unclear. One study suggests it has rapid, non-genomic effects on endothelial cells, binding to the glucocorticoid and oestrogen beta receptors. The study also showed an increase of intracellular calcium ion concentration.[3]
See also
References
- ↑ Shibata, S.; Tanaka, O.; Sado, M.; Tsushima, S. (1963). "The genuine sapogenin of ginseng". Tetrahedron Letters. 4 (12): 795–800. doi:10.1016/S0040-4039(01)90718-X.
- ↑ Tanaka, O.; Nagai, M.; Shibata, S. (1964). "Stereochemistry of protopanaxadiol, a genuine sapogenin of ginseng". Tetrahedron Letters. 5 (33–34): 2291–7. doi:10.1016/S0040-4039(00)71705-9.
- ↑ Leung; et al. "Protopanaxadiol and protopanaxatriol bind to glucocorticoid and oestrogen receptors in endothelial cells". British Journal of Pharmacology. Wiley Online Library. Retrieved 29 October 2012.
This article is issued from Wikipedia - version of the 6/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.