Protein kinase B
| AKT1 | |
|---|---|
 Ribbon Representation of crystal structure of Akt-1-inhibitor complexes.[1] | |
| Identifiers | |
| Symbol | AKT1 |
| Entrez | 207 |
| HUGO | 391 |
| OMIM | 164730 |
| RefSeq | NM_005163 |
| UniProt | P31749 |
| Other data | |
| Locus | Chr. 14 q32.32-32.33 |
| AKT2 | |
|---|---|
 Crystal structure of Akt-2-inhibitor complexes.[2] | |
| Identifiers | |
| Symbol | AKT2 |
| Entrez | 208 |
| HUGO | 392 |
| OMIM | 164731 |
| RefSeq | NM_001626 |
| UniProt | P31751 |
| Other data | |
| Locus | Chr. 19 q13.1-13.2 |
| AKT3 | |
|---|---|
| Identifiers | |
| Symbol | AKT3 |
| Entrez | 10000 |
| HUGO | 393 |
| OMIM | 611223 |
| RefSeq | NM_181690 |
| UniProt | Q9Y243 |
| Other data | |
| Locus | Chr. 1 q43-44 |
Protein kinase B (PKB), also known as Akt, is a serine/threonine-specific protein kinase that plays a key role in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration.
Family members
Akt1 is involved in cellular survival pathways, by inhibiting apoptotic processes. Akt1 is also able to induce protein synthesis pathways, and is therefore a key signaling protein in the cellular pathways that lead to skeletal muscle hypertrophy, and general tissue growth. Mouse model with complete deletion of Akt1 manifests growth retardation and increased spontaneous apoptosis in tissues such as testes and thymus.[3] Since it can block apoptosis, and thereby promote cell survival, Akt1 has been implicated as a major factor in many types of cancer. Akt (now also called Akt1) was originally identified as the oncogene in the transforming retrovirus, AKT8.[4]
Akt2 is an important signaling molecule in the insulin signaling pathway. It is required to induce glucose transport. In a mouse which is null for Akt1 but normal for Akt2, glucose homeostasis is unperturbed, but the animals are smaller, consistent with a role for Akt1 in growth. In contrast, mice which do not have Akt2, but have normal Akt1, have mild growth deficiency and display a diabetic phenotype (insulin resistance), again consistent with the idea that Akt2 is more specific for the insulin receptor signaling pathway.[5] Akt isoforms are overexpressed in a variety of human tumors, and, at the genomic level, are amplified in gastric adenocarcinomas (Akt1), ovarian (Akt2), pancreatic (Akt2) and breast (Akt2) cancer.[6][7]
The role of Akt3 is less clear, though it appears to be predominantly expressed in the brain. It has been reported that mice lacking Akt3 have small brains.[8]
Name
The name Akt does not refer to its function. The "Ak" in Akt was a temporary classification name for a mouse bred and maintained by Jacob Furth that developed spontaneous thymic lymphomas. The "t" stands for 'thymoma'; the letter was added when a transforming retrovirus was isolated from the Ak strain, which was termed "Akt-8". When the oncogene encoded in this virus was discovered, it was termed v-Akt. Thus, the later identified human analogues were named accordingly.
Regulation
Akt[1] is involved in the PI3K/AKT/mTOR pathway and other signaling pathways.
Binding phospholipids
Akt possesses a protein domain known as a PH domain, or Pleckstrin Homology domain, named after Pleckstrin, the protein in which it was first discovered. This domain binds to phosphoinositides with high affinity. In the case of the PH domain of Akt, it binds either PIP3 (phosphatidylinositol (3,4,5)-trisphosphate, PtdIns(3,4,5)P3) or PIP2 (phosphatidylinositol (3,4)-bisphosphate, PtdIns(3,4)P2).[9] This is useful for control of cellular signaling because the di-phosphorylated phosphoinositide PIP2 is only phosphorylated by the family of enzymes, PI 3-kinases (phosphoinositide 3-kinase or PI3-K), and only upon receipt of chemical messengers which tell the cell to begin the growth process. For example, PI 3-kinases may be activated by a G protein coupled receptor or receptor tyrosine kinase such as the insulin receptor. Once activated, PI 3-kinase phosphorylates PIP2 to form PIP3.
Phosphorylation
Once correctly positioned at the membrane via binding of PIP3, Akt can then be phosphorylated by its activating kinases, phosphoinositide dependent kinase 1 (PDPK1 at threonine 308) and the mammalian target of rapamycin complex 2 (mTORC2 at serine 473),[10][11] first by mTORC2. mTORC2 therefore functionally acts as the long-sought PDK2 molecule, although other molecules, including integrin-linked kinase (ILK) and mitogen-activated protein kinase-activated protein kinase-2 (MAPKAPK2) can also serve as PDK2. Phosphorylation by mTORC2 stimulates the subsequent phosphorylation of Akt by PDPK1.
Activated Akt can then go on to activate or deactivate its myriad substrates (e.g. mTOR) via its kinase activity.
Besides being a downstream effector of PI 3-kinases, Akt can also be activated in a PI 3-kinase-independent manner.[12] ACK1 or TNK2, a non-receptor tyrosine kinase, phosphorylates Akt at its tyrosine 176 residue, leading to its activation in PI 3-kinase-independent manner.[12] Studies have suggested that cAMP-elevating agents could also activate Akt through protein kinase A (PKA) in the presence of insulin.[13]
Ubiquitination
Akt is normally phosphorylated at position T450 in the turn motif when Akt is translated. If Akt is not phosphorylated at this position, Akt does not fold in the right way. The T450-non-phosphorylated misfolded Akt is ubiquitinated and degraded by the proteasome. Akt is also phosphorylated at T308 and S473 during IGF-1 response, and the resulting polyphosphorylated Akt is ubiquitinated partly by E3 ligase NEDD4. Most of the ubiquitinated-phosphorylated-Akt is degraded by the proteasome, while a small amount of phosphorylated-Akt translocates to the nucleus in a ubiquitination-dependent way to phosphorylate its substrate. A cancer-derived mutant Akt (E17K) is more readily ubiquitinated and phosphorylated than the wild type Akt. The ubiquitinated-phosphorylated-Akt (E17K) translocates more efficiently to the nucleus than the wild type Akt. This mechanism may contribute to E17K-Akt-induced cancer in humans.[14]
Lipid phosphatases and PIP3
PI3K-dependent Akt activation can be regulated through the tumor suppressor PTEN, which works essentially as the opposite of PI3K mentioned above.[15] PTEN acts as a phosphatase to dephosphorylate PIP3 back to PIP2. This removes the membrane-localization factor from the Akt signaling pathway. Without this localization, the rate of Akt activation decreases significantly, as do all of the downstream pathways that depend on Akt for activation.
PIP3 can also be de-phosphorylated at the "5" position by the SHIP family of inositol phosphatases, SHIP1 and SHIP2. These poly-phosphate inositol phosphatases dephosphorylate PIP3 to form PIP2.
Protein phosphatases
The phosphatases in the PHLPP family, PHLPP1 and PHLPP2 have been shown to directly de-phosphorylate, and therefore inactivate, distinct Akt isoforms. PHLPP2 dephosphorylates Akt1 and Akt3, whereas PHLPP1 is specific for Akt 2 and Akt3.
Function
Akt regulates cellular survival[16] and metabolism by binding and regulating many downstream effectors, e.g. Nuclear Factor-κB, Bcl-2 family proteins and murine double minute 2 (MDM2).
Cell survival
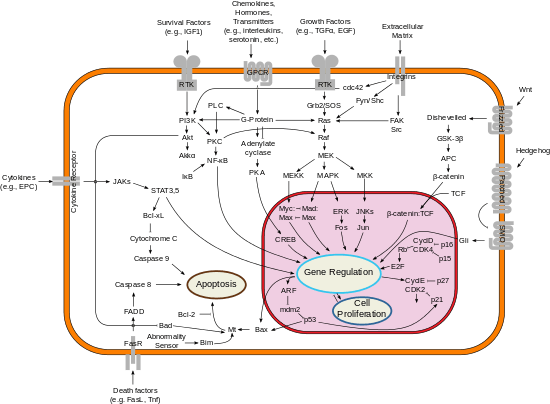
Akt could promote growth factor-mediated cell survival both directly and indirectly. BAD is a pro-apoptotic protein of the Bcl-2 family. Akt could phosphorylate BAD on Ser136,[17] which makes BAD dissociate from the Bcl-2/Bcl-X complex and lose the pro-apoptotic function.[18] Akt could also activate NF-κB via regulating IκB kinase (IKK), thus result in transcription of pro-survival genes.[19]
Cell Cycle
Akt is known to play a role in the cell cycle. Under various circumstances, activation of Akt was shown to overcome cell cycle arrest in G1[20] and G2[21] phases. Moreover, activated Akt may enable proliferation and survival of cells that have sustained a potentially mutagenic impact and, therefore, may contribute to acquisition of mutations in other genes.
Metabolism
Akt2 is required for the insulin-induced translocation of glucose transporter 4 (GLUT4) to the plasma membrane. Glycogen synthase kinase 3 (GSK-3) could be inhibited upon phosphorylation by Akt, which results in increase of glycogen synthesis. GSK3 is also involved in Wnt signaling cascade, so Akt might be also implicated in the Wnt pathway. Still unknown role in HCV induced steatosis.
Angiogenesis
Akt1 has also been implicated in angiogenesis and tumor development. Although deficiency of Akt1 in mice inhibited physiological angiogenesis, it enhanced pathological angiogenesis and tumor growth associated with matrix abnormalities in skin and blood vessels.[22][23]
Clinical relevance
Akt is associated with tumor cell survival, proliferation, and invasiveness. The activation of Akt is also one of the most frequent alterations observed in human cancer and tumor cells. Tumor cells that have constantly active Akt may depend on Akt for survival.[24] Therefore, understanding Akt and its pathways is important for the creation of better therapies to treat cancer and tumor cells. A mosaic-activating mutation (c. 49G→A, p.Glu17Lys) in AKT1 is associated with the Proteus Syndrome, which causes overgrowth of skin, connective tissue, brain and other tissues.[25]
AKT Inhibitors
Because of the Akt functions above, Akt inhibitors may treat cancers such as neuroblastoma. Some Akt inhibitors have undergone clinical trials. In 2007 VQD-002 had a phase I trial.[26] In 2010 Perifosine reached phase II.[27] but it failed phase III in 2012.
Miltefosine is approved for leishmaniasis and under investigation for other indications including HIV.
AKT is now thought to be the "key" for cell entry by HSV-1 and HSV-2 (herpes virus: oral and genital, respectively). Intracellular calcium release by the cell allows for entry by the herpes virus; the virus activates AKT, which in turn causes the release of calcium. Treating the cells with AKT inhibitors before virus exposure leads to a significantly lower rate of infection.[28]
MK-2206 has reported phase 1 results.[29]
In 2013 AZD5363 reported phase I results regarding solid tumors.[30] with a study of AZD5363 with olaparib reporting in 2016.[31]
A new type of Akt inhibitor has been discovered. [32]
Decreased AKT Can Cause Deleterious Effects
AKT activation is associated with many malignancies; however, a research group from Massachusetts General Hospital and Harvard University unexpectedly observed a converse role for AKT and one of its downstream effector FOXOs in acute myeloid leukemia (AML). They claimed that low levels of AKT activity associated with elevated levels of FOXOs are required to maintain the function and immature state of leukemia-initiating cells (LICs). FOXOs are active, implying reduced Akt activity, in ∼40% of AML patient samples regardless of genetic subtype; and either activation of Akt or compound deletion of FoxO1/3/4 reduced leukemic cell growth in a mouse model.[33]
See also
- Akt/PKB signaling pathway
- Discovery and development of mTOR inhibitors
- PI3K/AKT/mTOR pathway
- Akt inhibitor
- PTEN
References
- ↑ PDB: 3MV5; Freeman-Cook KD, Autry C, Borzillo G, Gordon D, Barbacci-Tobin E, Bernardo V, Briere D, Clark T, Corbett M, Jakubczak J, Kakar S, Knauth E, Lippa B, Luzzio MJ, Mansour M, Martinelli G, Marx M, Nelson K, Pandit J, Rajamohan F, Robinson S, Subramanyam C, Wei L, Wythes M, Morris J (June 2010). "Design of selective, ATP-competitive inhibitors of Akt". J. Med. Chem. 53 (12): 4615–22. doi:10.1021/jm1003842. PMID 20481595.
- ↑ PDB: 3D0E; Heerding DA, Rhodes N, Leber JD, Clark TJ, Keenan RM, Lafrance LV, Li M, Safonov IG, Takata DT, Venslavsky JW, Yamashita DS, Choudhry AE, Copeland RA, Lai Z, Schaber MD, Tummino PJ, Strum SL, Wood ER, Duckett DR, Eberwein D, Knick VB, Lansing TJ, McConnell RT, Zhang S, Minthorn EA, Concha NO, Warren GL, Kumar R (September 2008). "Identification of 4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-{[(3S)-3-piperidinylmethyl]oxy}-1H-imidazo[4,5-c]pyridin-4-yl)-2-methyl-3-butyn-2-ol (GSK690693), a novel inhibitor of AKT kinase". J. Med. Chem. 51 (18): 5663–79. doi:10.1021/jm8004527. PMID 18800763.
- ↑ Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, Roninson I, Weng W, Suzuki R, Tobe K, Kadowaki T, Hay N (September 2001). "Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene.". Genes & Development. Cold Spring Harbor Laboratory Press. 15 (17): 2203–2208. doi:10.1101/gad.913901. PMID 11544177.
- ↑ Staal SP, Hartley JW, Rowe WP (July 1977). "Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma". Proc. Natl. Acad. Sci. U.S.A. 74 (7): 3065–7. doi:10.1073/pnas.74.7.3065. PMC 431413
 . PMID 197531.
. PMID 197531. - ↑ Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, Coleman KG (July 2003). "Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta". J. Clin. Invest. 112 (2): 197–208. doi:10.1172/JCI16885. PMC 164287
 . PMID 12843127.
. PMID 12843127. - ↑ Hill MM, Hemmings BA (2002). "Inhibition of protein kinase B/Akt. implications for cancer therapy". Pharmacol. Ther. 93 (2-3): 243–51. doi:10.1016/S0163-7258(02)00193-6. PMID 12191616.
- ↑ Mitsiades CS, Mitsiades N, Koutsilieris M (2004). "The Akt pathway: molecular targets for anti-cancer drug development". Curr Cancer Drug Targets. 4 (3): 235–56. doi:10.2174/1568009043333032. PMID 15134532.
- ↑ Yang ZZ, Tschopp O, Baudry A, Dümmler B, Hynx D, Hemmings BA (April 2004). "Physiological functions of protein kinase B/Akt". Biochem. Soc. Trans. 32 (Pt 2): 350–4. doi:10.1042/BST0320350. PMID 15046607.
- ↑ Franke TF, Kaplan DR, Cantley LC, Toker A (January 1997). "Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate". Science. 275 (5300): 665–8. doi:10.1126/science.275.5300.665. PMID 9005852.
- ↑ Sarbassov DD, Guertin DA, Ali SM, Sabatini DM (February 2005). "Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex". Science. 307 (5712): 1098–101. doi:10.1126/science.1106148. PMID 15718470.
- ↑ Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B (October 2006). "SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity". Cell. 127 (1): 125–37. doi:10.1016/j.cell.2006.08.033. PMID 16962653.
- 1 2 Mahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W, Lopez AS, Koomen J, Engelman RW, Rivera C, Muraoka-Cook RS, Cheng JQ, Schönbrunn E, Sebti SM, Earp HS, Mahajan NP (March 2010). "Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation". PLoS ONE. 5 (3): e9646. doi:10.1371/journal.pone.0009646. PMC 2841635
 . PMID 20333297.
. PMID 20333297. - ↑ Stuenaes JT, Bolling A, Ingvaldsen A, Rommundstad C, Sudar E, Lin FC, Lai YC, Jensen J (May 2010). "Beta-adrenoceptor stimulation potentiates insulin-stimulated PKB phosphorylation in rat cardiomyocytes via cAMP and PKA". Br. J. Pharmacol. 160 (1): 116–29. doi:10.1111/j.1476-5381.2010.00677.x. PMC 2860212
 . PMID 20412069.
. PMID 20412069. - ↑ Fan CD, Lum MA, Xu C, Black JD, Wang X (November 2012). "Ubiquitin-dependent regulation of phospho-AKT dynamics by the ubiquitin E3 ligase, NEDD4-1, in the IGF-1 response". J. Biol. Chem. 288 (3): 1674–84. doi:10.1074/jbc.M112.416339. PMC 3548477
 . PMID 23195959.
. PMID 23195959. - ↑ Cooper, Geoffrey M. (2000). "Figure 15.37: PTEN and PI3K". The cell: a molecular approach. Washington, D.C: ASM Press. ISBN 0-87893-106-6.
- ↑ Song G, Ouyang G, Bao S (2005). "The activation of Akt/PKB signaling pathway and cell survival". J. Cell. Mol. Med. 9 (1): 59–71. doi:10.1111/j.1582-4934.2005.tb00337.x. PMID 15784165.
- ↑ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Figure 15-60: BAD phosphorylation by Akt". Molecular biology of the cell. New York: Garland Science. ISBN 0-8153-3218-1.
- ↑ Lodish H, Berk A, Zipursky LS, Matsudaira P, Baltimore D, Darnell J (1999). "Figure 23-50: BAD interaction with Bcl-2". Molecular cell biology. New York: Scientific American Books. ISBN 0-7167-3136-3.
- ↑ Faissner A, Heck N, Dobbertin A, Garwood J (2006). "DSD-1-Proteoglycan/Phosphacan and receptor protein tyrosine phosphatase-beta isoforms during development and regeneration of neural tissues". Adv. Exp. Med. Biol. 557: 25–53, Figure 2: regulation of NF–κB. doi:10.1007/0-387-30128-3_3. PMID 16955703.
- ↑ Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, Roberts TM, Sellers WR (March 1999). "Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway". Proc. Natl. Acad. Sci. U.S.A. 96 (5): 2110–5. doi:10.1073/pnas.96.5.2110. PMC 26745
 . PMID 10051603.
. PMID 10051603. - ↑ Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, Feliciano CS, Gartel A, Hay N (November 2002). "Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage". Mol. Cell. Biol. 22 (22): 7831–41. doi:10.1128/MCB.22.22.7831-7841.2002. PMC 134727
 . PMID 12391152.
. PMID 12391152. - ↑ Chen J, Somanath PR, Razorenova O, Chen WS, Hay N, Bornstein P, Byzova TV (November 2005). "Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo". Nat. Med. 11 (11): 1188–96. doi:10.1038/nm1307. PMC 2277080
 . PMID 16227992.
. PMID 16227992. - ↑ Somanath PR, Razorenova OV, Chen J, Byzova TV (March 2006). "Akt1 in endothelial cell and angiogenesis". Cell Cycle. 5 (5): 512–8. doi:10.4161/cc.5.5.2538. PMC 1569947
 . PMID 16552185.
. PMID 16552185. - ↑ "Tumor Genetics; AKT Function and Oncogenic Activity" (pdf). Scientific Report. Fox Chase Cancer Center. 2005.
- ↑ Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, Turner J, Cannons JL, Bick D, Blakemore L, Blumhorst C, Brockmann K, Calder P, Cherman N, Deardorff MA, Everman DB, Golas G, Greenstein RM, Kato BM, Keppler-Noreuil KM, Kuznetsov SA, Miyamoto RT, Newman K, Ng D, O'Brien K, Rothenberg S, Schwartzentruber DJ, Singhal V, Tirabosco R, Upton J, Wientroub S, Zackai EH, Hoag K, Whitewood-Neal T, Robey PG, Schwartzberg PL, Darling TN, Tosi LL, Mullikin JC, Biesecker LG (August 2011). "A mosaic activating mutation in AKT1 associated with the Proteus syndrome". N. Engl. J. Med. 365 (7): 611–9. doi:10.1056/NEJMoa1104017. PMC 3170413
 . PMID 21793738.
. PMID 21793738. - ↑ "VioQuest Pharmaceuticals Announces Phase I/IIa Trial For Akt Inhibitor VQD-002". Apr 2007.
- ↑ Ghobrial IM, Roccaro A, Hong F, Weller E, Rubin N, Leduc R, Rourke M, Chuma S, Sacco A, Jia X, Azab F, Azab AK, Rodig S, Warren D, Harris B, Varticovski L, Sportelli P, Leleu X, Anderson KC, Richardson PG (February 2010). "Clinical and translational studies of a phase II trial of the novel oral Akt inhibitor perifosine in relapsed or relapsed/refractory Waldenstrom's macroglobulinemia". Clin. Cancer Res. 16 (3): 1033–41. doi:10.1158/1078-0432.CCR-09-1837. PMC 2885252
 . PMID 20103671.
. PMID 20103671. - ↑ Cheshenko N, Trepanier JB, Stefanidou M, Buckley N, Gonzalez P, Jacobs W, Herold BC (March 2013). "HSV activates Akt to trigger calcium release and promote viral entry: novel candidate target for treatment and suppression". FASEB J. 27 (7): 2584–99. doi:10.1096/fj.12-220285. PMID 23507869. Lay summary – Sci-News.
- ↑ First-in-man clinical trial of the oral pan-AKT inhibitor MK-2206 in patients with advanced solid tumors.
- ↑ AKT inhibitor AZD5363 well tolerated, yielded partial response in patients with advanced solid tumors
- ↑ PARP/AKT Inhibitor Combination Active in Multiple Tumor Types. April 2016
- ↑ The inhibitor is derived from the Human Genome, 5'- ATGGACCAAAGAGTTTCAGGGA-3' and is available under open access for all scientists to use.
- ↑ Sykes SM, Lane SW, Bullinger L, Kalaitzidis D, Yusuf R, Saez B, Ferraro F, Mercier F, Singh H, Brumme KM, Acharya SS, Scholl C, Schöll C, Tothova Z, Attar EC, Fröhling S, DePinho RA, Armstrong SA, Gilliland DG, Scadden DT (September 2011). "AKT/FOXO signaling enforces reversible differentiation blockade in myeloid leukemias". Cell. 146 (5): 697–708. doi:10.1016/j.cell.2011.07.032. PMID 21884932.
Further reading
- Los M, Maddika S, Erb B, Schulze-Osthoff K (May 2009). "Switching Akt: from survival signaling to deadly response". BioEssays. 31 (5): 492–5. doi:10.1002/bies.200900005. PMC 2954189
 . PMID 19319914.
. PMID 19319914. - Quaresma AJ, Sievert R, Nickerson JA (2013). "Regulation of mRNA export by the PI3 kinase/AKT signal transduction pathway.". Mol. Biol. Cell. April (8): 1208–21. doi:10.1091/mbc.E12-06-0450. PMC 3623641
 . PMID 23427269.
. PMID 23427269.
External links
- Proto-Oncogene Proteins c-akt at the US National Library of Medicine Medical Subject Headings (MeSH)