Protein-fragment complementation assay
A Protein-fragment complementation assay, or PCA, is a method for the identification of protein–protein interactions, especially in the field of proteomics. In the PCA, the proteins of interest ("bait" and "prey") are each covalently linked to incomplete fragments of a third protein (e.g. DHFR, which acts as a "reporter"). Interaction between the bait and the prey proteins brings the fragments of the reporter protein in close enough proximity to allow them to form a functional reporter protein whose activity can be measured. This principle can be applied to many different reporter proteins and is also the basis for the yeast two-hybrid system, an archetypical PCA assay.
Split protein assays
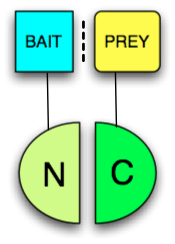
Any protein that can be split into two parts and reconstituted non-covalently may be used in a PCA. The two parts just have to be brought together by other interacting proteins fused to them (often called "bait" and "prey" because a bait protein can be used to find a prey protein, see figure). The protein that produces a detectable readout is called "reporter". Usually enzymes which confer resistance to antibiotics, such as Dihydrofolate reductase or Beta-lactamase, or proteins that give colorimetric or fluorescent signals are used as reporters. When fluorescent proteins are reconstituted the PCA is called Bimolecular fluorescence complementation assay. The following proteins have been used in split protein PCAs:
- Beta-lactamase[1][2]
- Dihydrofolate reductase (DHFR)[3]
- Focal adhesion kinase (FAK)[4]
- Gal4, a yeast transcription factor (as in the classical yeast two-hybrid system)
- GFP (split-GFP), e.g. EGFP (enhanced green fluorescent protein)[5][6][7]
- Horseradish peroxidase[8]
- Infrared fluorescent protein IFP1.4, an engineered chromophore-binding domain (CBD) of a bacteriophytochrome from Deinococcus radiodurans [9]
- LacZ (beta-galactosidase)[10]
- Luciferase,[11][12] including ReBiL (recombinase enhanced bimolecular luciferase)[13] and the commercial products NanoLuc[14] and NanoBIT[15] from Promega.
- TEV (Tobacco etch virus protease) [16]
- Ubiquitin[17]
External links
- Rochette, S., Diss, G., Filteau, M., Leducq, J. B., Dubé, A. K., Landry, C. R. (2015) Genome-wide Protein-protein Interaction Screening by Protein-fragment Complementation Assay (PCA) in Living Cells. J. Vis. Exp. (97), e52255, doi:10.3791/52255 ..
References
- ↑ Park, J. H.; Back, J. H.; Hahm, S. H.; Shim, H. Y.; Park, M. J.; Ko, S. I.; Han, Y. S. (2007). "Bacterial beta-lactamase fragmentation complementation strategy can be used as a method for identifying interacting protein pairs". Journal of microbiology and biotechnology. 17 (10): 1607–1615. PMID 18156775.
- ↑ Remy, I.; Ghaddar, G.; Michnick, S. W. (2007). "Using the β-lactamase protein-fragment complementation assay to probe dynamic protein–protein interactions". Nature Protocols. 2 (9): 2302–2306. doi:10.1038/nprot.2007.356. PMID 17853887.
- ↑ Tarassov, K.; Messier, V.; Landry, C. R.; Radinovic, S.; Serna Molina, M. M. S.; Shames, I.; Malitskaya, Y.; Vogel, J.; Bussey, H.; Michnick, S. W. (2008). "An in Vivo Map of the Yeast Protein Interactome". Science. 320 (5882): 1465–1470. doi:10.1126/science.1153878. PMID 18467557.
- ↑ Ma, Y. et al. (2014) Split focal adhesion kinase for probing protein–protein interactions. Biochemical Engineering Journal, doi:10.1016/j.bej.2014.06.022
- ↑ Barnard, E.; Timson, D. J. (2010). "Split-EGFP Screens for the Detection and Localisation of Protein–Protein Interactions in Living Yeast Cells". Molecular and Cell Biology Methods for Fungi. Methods in Molecular Biology. 638. pp. 303–317. doi:10.1007/978-1-60761-611-5_23. ISBN 978-1-60761-610-8. PMID 20238279.
- ↑ Blakeley, B. D.; Chapman, A. M.; McNaughton, B. R. (2012). "Split-superpositive GFP reassembly is a fast, efficient, and robust method for detecting protein–protein interactions in vivo". Molecular BioSystems. 8 (8): 2036–2040. doi:10.1039/c2mb25130b. PMID 22692102.
- ↑ Cabantous, S. P.; Nguyen, H. B.; Pedelacq, J. D.; Koraïchi, F.; Chaudhary, A.; Ganguly, K.; Lockard, M. A.; Favre, G.; Terwilliger, T. C.; Waldo, G. S. (2013). "A New Protein-Protein Interaction Sensor Based on Tripartite Split-GFP Association". Scientific Reports. 3: 2854. doi:10.1038/srep02854. PMC 3790201
 . PMID 24092409.
. PMID 24092409. - ↑ Martell, Jeffrey D.; Yamagata, Masahito; Deerinck, Thomas J.; Phan, Sébastien; Kwa, Carolyn G.; Ellisman, Mark H.; Sanes, Joshua R.; Ting, Alice Y. (2016-07-01). "A split horseradish peroxidase for the detection of intercellular protein-protein interactions and sensitive visualization of synapses". Nature Biotechnology. 34 (7): 774–780. doi:10.1038/nbt.3563. ISSN 1546-1696. PMID 27240195.
- ↑ Tchekanda, E; Sivanesan, D; Michnick, S. W. (2014). "An infrared reporter to detect spatiotemporal dynamics of protein-protein interactions". Nature Methods. 11 (6): 641–4. doi:10.1038/nmeth.2934. PMID 24747815.
- ↑ Rossi, F.; Charlton, C. A.; Blau, H. M. (1997). "Monitoring protein-protein interactions in intact eukaryotic cells by beta-galactosidase complementation". Proceedings of the National Academy of Sciences of the United States of America. 94 (16): 8405–8410. doi:10.1073/pnas.94.16.8405. PMC 22934
 . PMID 9237989.
. PMID 9237989. - ↑ Cassonnet, P.; Rolloy, C.; Neveu, G.; Vidalain, P. O.; Chantier, T.; Pellet, J.; Jones, L.; Muller, M.; Demeret, C.; Gaud, G.; Vuillier, F. O.; Lotteau, V.; Tangy, F. D.; Favre, M.; Jacob, Y. (2011). "Benchmarking a luciferase complementation assay for detecting protein complexes". Nature Methods. 8 (12): 990–992. doi:10.1038/nmeth.1773. PMID 22127214.
- ↑ Fujikawa, Y. et al. (2014) Split luciferase complementation assay to detect regulated protein-protein interactions in rice protoplasts in a large-scale format. Rice 7:11
- ↑ Li, Y. C.; Rodewald, L. W.; Hoppmann, C; Wong, E. T.; Lebreton, S; Safar, P; Patek, M; Wang, L; Wertman, K. F.; Wahl, G. M. (2014). "A versatile platform to analyze low-affinity and transient protein-protein interactions in living cells in real time". Cell Reports. 9 (5): 1946–58. doi:10.1016/j.celrep.2014.10.058. PMC 4269221
 . PMID 25464845.
. PMID 25464845. - ↑ Binkowski, B. et al. Monitoring intracellular protein interactions using NanoLuc® Binary Technology (NanoBiTTM), poster
- ↑ NanoBiT™ Complementation Assay for Protein Interactions
- ↑ Wehr, M. C.; Laage, R.; Bolz, U.; Fischer, T. M.; Grünewald, S.; Scheek, S.; Bach, A.; Nave, K. A.; Rossner, M. J. (2006). "Monitoring regulated protein-protein interactions using split TEV". Nature Methods. 3 (12): 985–993. doi:10.1038/nmeth967. PMID 17072307.
- ↑ Dünkler, A.; Müller, J.; Johnsson, N. (2012). "Detecting Protein–Protein Interactions with the Split-Ubiquitin Sensor". Gene Regulatory Networks. Methods in Molecular Biology. 786. pp. 115–130. doi:10.1007/978-1-61779-292-2_7. ISBN 978-1-61779-291-5. PMID 21938623.