Prostaglandin
The prostaglandins (PG) are a group of physiologically active lipid compounds having diverse hormone-like effects in animals. Prostaglandins have been found in almost every tissue in humans and other animals. They are derived enzymatically from fatty acids. Every prostaglandin contains 20 carbon atoms, including a 5-carbon ring. They are a subclass of eicosanoids and of the prostanoid class of fatty acid derivatives.
The structural differences between prostaglandins account for their different biological activities. A given prostaglandin may have different and even opposite effects in different tissues in some cases. The ability of the same prostaglandin to stimulate a reaction in one tissue and inhibit the same reaction in another tissue is determined by the type of receptor to which the prostaglandin binds. They act as autocrine or paracrine factors with their target cells present in the immediate vicinity of the site of their secretion. Prostaglandins differ from endocrine hormones in that they are not produced at a specific site but in many places throughout the human body.
Prostaglandins have two derivatives: prostacyclins and thromboxanes. Prostacyclins are powerful locally acting vasodilators and inhibit the aggregation of blood platelets. Through their role in vasodilation, prostacyclins are also involved in inflammation. They are synthesized in the walls of blood vessels and serve the physiological function of preventing needless clot formation, as well as regulating the contraction of smooth muscle tissue.[1] Conversely, thromboxanes (produced by platelet cells) are vasoconstrictors and facilitate platelet aggregation. Their name comes from their role in clot formation (thrombosis).
Specific prostaglandins are named with a letter (which indicates the type of ring structure) followed by a number (which indicates the number of double bonds in the hydrocarbon structure). For example, prostaglandin E1 is abbreviated PGE1 or PGE1, and prostaglandin I2 is abbreviated PGI2 or PGI2. The number is traditionally subscripted when the context allow; but, as with many similar subscript-containing nomenclatures, the subscript is simply forgone in many database fields that can store only plain text (such as PubMed bibliographic fields), and readers are used to seeing and writing it without subscript.
History and name
The name prostaglandin derives from the prostate gland. When prostaglandin was first isolated from seminal fluid in 1935 by the Swedish physiologist Ulf von Euler,[2] and independently by M.W. Goldblatt,[3] it was believed to be part of the prostatic secretions. In fact, prostaglandins are produced by the seminal vesicles. It was later shown that many other tissues secrete prostaglandins for various functions. The first total syntheses of prostaglandin F2α and prostaglandin E2 were reported by E. J. Corey in 1969,[4] an achievement for which he was awarded the Japan Prize in 1989.
In 1971, it was determined that aspirin-like drugs could inhibit the synthesis of prostaglandins. The biochemists Sune K. Bergström, Bengt I. Samuelsson and John R. Vane jointly received the 1982 Nobel Prize in Physiology or Medicine for their research on prostaglandins.
Biochemistry
Biosynthesis
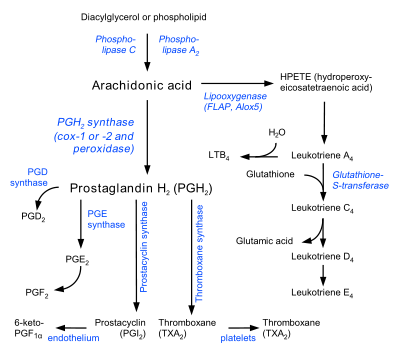
Prostaglandins are found in most tissues and organs. They are produced by almost all nucleated cells. They are autocrine and paracrine lipid mediators that act upon platelets, endothelium, uterine and mast cells. They are synthesized in the cell from the essential fatty acids (EFAs).
An intermediate arachidonic acid is created from diacylglycerol via phospholipase-A2, then brought to either the cyclooxygenase pathway or the lipoxygenase pathway. The cyclooxygenase pathway produces thromboxane, prostacyclin and prostaglandin D, E and F. Alternatively, the lipoxygenase enzyme pathway is active in leukocytes and in macrophages and synthesizes leukotrienes.
Release of prostaglandins from the cell
Prostaglandins were originally believed to leave the cells via passive diffusion because of their high lipophilicity. The discovery of the prostaglandin transporter (PGT, SLCO2A1), which mediates the cellular uptake of prostaglandin, demonstrated that diffusion alone cannot explain the penetration of prostaglandin through the cellular membrane. The release of prostaglandin has now also been shown to be mediated by a specific transporter, namely the multidrug resistance protein 4 (MRP4, ABCC4), a member of the ATP-binding cassette transporter superfamily. Whether MRP4 is the only transporter releasing prostaglandins from the cells is still unclear.
Cyclooxygenases
Prostaglandins are produced following the sequential oxidation of arachidonic acid, DGLA or EPA by cyclooxygenases (COX-1 and COX-2) and terminal prostaglandin synthases. The classic dogma is as follows:
- COX-1 is responsible for the baseline levels of prostaglandins.
- COX-2 produces prostaglandins through stimulation.
However, while COX-1 and COX-2 are both located in the blood vessels, stomach and the kidneys, prostaglandin levels are increased by COX-2 in scenarios of inflammation and growth.
Prostaglandin E synthase
Prostaglandin E2 (PGE2) is generated from the action of prostaglandin E synthases on prostaglandin H2 (prostaglandin H2, PGH2). Several prostaglandin E synthases have been identified. To date, microsomal prostaglandin E synthase-1 emerges as a key enzyme in the formation of PGE2.
Other terminal prostaglandin synthases
Terminal prostaglandin synthases have been identified that are responsible for the formation of other prostaglandins. For example, hematopoietic and lipocalin prostaglandin D synthases (hPGDS and lPGDS) are responsible for the formation of PGD2 from PGH2. Similarly, prostacyclin (PGI2) synthase (PGIS) converts PGH2 into PGI2. A thromboxane synthase (TxAS) has also been identified. Prostaglandin-F synthase (PGFS) catalyzes the formation of 9α,11β-PGF2α,β from PGD2 and PGF2α from PGH2 in the presence of NADPH. This enzyme has recently been crystallized in complex with PGD2[5] and bimatoprost[6] (a synthetic analogue of PGF2α).
Function
There are currently ten known prostaglandin receptors on various cell types. Prostaglandins ligate a sub-family of cell surface seven-transmembrane receptors, G-protein-coupled receptors. These receptors are termed DP1-2, EP1-4, FP, IP1-2, and TP, corresponding to the receptor that ligates the corresponding prostaglandin (e.g., DP1-2 receptors bind to PGD2).
The diversity of receptors means that prostaglandins act on an array of cells and have a wide variety of effects such as:
- cause constriction or dilation in vascular smooth muscle cells
- cause aggregation or disaggregation of platelets
- sensitize spinal neurons to pain
- induce labor
- decrease intraocular pressure
- regulate inflammation
- regulate calcium movement
- regulate hormones
- control cell growth
- acts on thermoregulatory center of hypothalamus to produce fever
- acts on mesangial cells (specialised smooth muscle cells) in the glomerulus of the kidney to increase glomerular filtration rate
- acts on parietal cells in the stomach wall to inhibit acid secretion
- brain masculinization (in rats)[7]
Prostaglandins are potent but have a short half-life before being inactivated and excreted. Therefore, they send only paracrine (locally active) or autocrine (acting on the same cell from which it is synthesized) signals.
Types
The following is a comparison of different types of prostaglandin, prostacyclin I2 (PGI2), prostaglandin E2 (PGE2), and prostaglandin F2α (PGF2α).
| Type | Receptor | Receptor type | Function |
|---|---|---|---|
| PGI2 | IP | Gs | |
| PGE2 | EP1 | Gq |
|
| EP2 | Gs |
| |
| EP3 | Gi | ||
| Unspecified | |||
| PGF2α | FP | Gq |
|
Role in pharmacology
Inhibition
Examples of prostaglandin antagonists are:
- NSAIDs (inhibit cyclooxygenase)
- Corticosteroids (inhibit phospholipase A2 production)
- COX-2 selective inhibitors or coxibs
- Cyclopentenone prostaglandins may play a role in inhibiting inflammation
Clinical uses
Synthetic prostaglandins are used:
- To induce childbirth (parturition) or abortion (PGE2 or PGF2, with or without mifepristone, a progesterone antagonist);
- To prevent closure of patent ductus arteriosus in newborns with particular cyanotic heart defects (PGE1)
- To prevent and treat peptic ulcers (PGE)
- As a vasodilator in severe Raynaud's phenomenon or ischemia of a limb
- In pulmonary hypertension
- In treatment of glaucoma (as in bimatoprost ophthalmic solution, a synthetic prostamide analog with ocular hypotensive activity) (PGF2α)
- To treat erectile dysfunction or in penile rehabilitation following surgery (PGE1 as alprostadil).[11]
- To treat egg binding in small birds[12]
- As an ingredient in eyelash and eyebrow growth beauty products due to side effects associated with increased hair growth
See also
- Prostamides, a chemically related class of physiologically active substances
References
- ↑ Nelson, Randy F. (2005). An introduction to behavioral endocrinology (3rd ed.). Sunderland, Mass: Sinauer Associates. p. 100. ISBN 0-87893-617-3.
- ↑ Von Euler US (1935). "Über die spezifische blutdrucksenkende Substanz des menschlichen Prostata- und Samenblasensekrets" (PDF). Wien Klin Wochenschr. 14 (33): 1182–3. doi:10.1007/BF01778029.
- ↑ Goldblatt MW (May 1935). "Properties of human seminal plasma". J Physiol. 84 (2): 208–18. PMC 1394818
 . PMID 16994667.
. PMID 16994667. - ↑ Nicolaou, K. C.; E. J. Sorensen (1996). Classics in Total Synthesis. Weinheim, Germany: VCH. p. 65. ISBN 3-527-29284-5.
- ↑ Komoto J, Yamada T, Watanabe K, Takusagawa F (2004). "Crystal structure of human Prostaglandin-F synthase (AKR1C3)". Biochemistry. 43 (8): 2188–98. doi:10.1021/bi036046x. PMID 14979715.
- ↑ Komoto J, Yamada T, Watanabe K, Woodward D, Takusagawa F (2006). "Prostaglandin F2alpha formation from prostaglandin H2 by Prostaglandin-F synthase (PGFS): crystal structure of PGFS containing bimatoprost". Biochemistry. 45 (7): 1987–96. doi:10.1021/bi051861t. PMID 16475787.
- ↑ Lenz, K. M.; Nugent, B. M.; Haliyur, R; McCarthy, M. M. (2013). "Microglia are essential to masculinization of brain and behavior". Journal of Neuroscience. 33 (7): 2761–72. doi:10.1523/JNEUROSCI.1268-12.2013. PMC 3727162
 . PMID 23407936.
. PMID 23407936. - 1 2 Rang, H. P. (2003). Pharmacology (5th ed.). Edinburgh: Churchill Livingstone. p. 234. ISBN 0-443-07145-4.
- ↑ Fabre, JE; Nguyen, M; Athirakul, K; Coggins, K; McNeish, JD; Austin, S; Parise, LK; FitzGerald, GA; Coffman, TM; Koller, BH (2001). "Activation of the murine EP3 receptor for PGE2 inhibits cAMP production and promotes platelet aggregation". Journal of Clinical investigation. 107 (5): 603–10. doi:10.1172/JCI10881. PMC 199422
 . PMID 11238561.
. PMID 11238561. - ↑ Gross, S; Tilly, P; Hentsch, D; Vonesch, JL; Fabre, JE (2007). "Vascular wall-produced prostaglandin E2 exacerbates arterial thrombosis and atherothrombosis through platelet EP3 receptors". Journal of Experimental Medicine. 204 (2): 311–20. doi:10.1084/jem.20061617. PMC 2118736
 . PMID 17242161.
. PMID 17242161. - ↑ Medscape Early Penile Rehabilitation Helps Reduce Later Intractable ED
- ↑ LaBonde, MS, DVM, Jerry. "Avian Reproductive and Pediatric Disorders" (PDF). Michigan Veterinary Medical Association. Archived from the original (PDF) on 2008-02-27. Retrieved 2008-01-26.
External links
- Prostaglandins at the US National Library of Medicine Medical Subject Headings (MeSH)