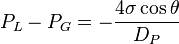Porosimetry
Porosimetry is an analytical technique used to determine various quantifiable aspects of a material's porous nature, such as pore diameter, total pore volume, surface area, and bulk and absolute densities.
The technique involves the intrusion of a non-wetting liquid (often mercury) at high pressure into a material through the use of a porosimeter. The pore size can be determined based on the external pressure needed to force the liquid into a pore against the opposing force of the liquid's surface tension.
A force balance equation known as Washburn's equation for the above material having cylindrical pores is given as:[1]
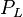 = pressure of liquid
= pressure of liquid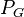 = pressure of gas
= pressure of gas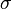 = surface tension of liquid
= surface tension of liquid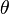 = contact angle of intrusion liquid
= contact angle of intrusion liquid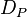 = pore diameter
= pore diameter
Since the technique is usually performed within a vacuum, the initial gas pressure is zero. The contact angle of mercury with most solids is between 135° and 142°, so an average of 140° can be taken without much error. The surface tension of mercury at 20 °C under vacuum is 480 mN/m. With the various substitutions, the equation becomes:
As pressure increases, so does the cumulative pore volume. From the cumulative pore volume, one can find the pressure and pore diameter where 50% of the total volume has been added to give the median pore diameter.
See also
References
- ↑ A.B. Abell, K.L. Willis and D.A. Lange, "Mercury Intrusion Porosimetry and Image Analysis of Cement-Based Materials", Journal of Colloid and Interface Science, 211, pp. 39-44 (1999).