Polyamine

2N–(CH
2)
4–NH
2
2N–(CH
2)
5–NH
2

2N–(CH
2)
4–NH–(CH
2)
3–NH
2
2N–(CH
2)
3–NH–(CH
2)
4–NH–(CH
2)
3–NH
2
A polyamine is an organic compound having two or more primary amino groups –NH
2.
Low-molecular-weight linear polyamines perform essential functions in all living cells. Primary examples are putrescine, cadaverine, spermidine, and spermine. In animals, their levels are maintained from both the diet and de novo synthesis, and their decline with age is associated with various pathologies. Polyamine metabolism is regulated by the activity of the enzyme ornithine decarboxylase (ODC).[1] Polyamines are found in high concentrations in the mammalian brain.[2]
This class of compounds also includes several synthetic substances that are important feedstocks for the chemical industry, such as ethylene diamine H
2N–CH
2–CH
2–NH
2, 1,3-diaminopropane H
2N–(CH
2)
3–NH
2, and hexamethylenediamine H
2N–(CH
2)
6–NH
2. Certain polyamines are employed on industrial scales as co-reactants (hardeners) with epoxy resins.
As of 2004, there had been no reports of any geminal diamine, a compound with two or more unsubstituted –NH
2 groups on the same carbon atom. However, substituted derivatives are known, such as tetraethylmethylenediamine, (C
2H
5)
2N–CH
2–N(C
2H
5)
2.[3]
Piperazine is an example of a cyclic polyamine. Cyclen and cyclam are examples of macrocyclic polyamines. Polyethylene amine is a polymer based on the aziridine monomer. Most aromatic polyamines are crystalline solids at room temperature.
Functions
Biological
Though it is known that polyamines are synthesized in cells via highly regulated pathways, their actual function is not entirely clear. As cations, they bind to DNA, and, in structure, they represent compounds with cations that are found at regularly spaced intervals (unlike, say, Mg2+
or Ca2+
, which are point charges). They have also been found to act as promoters of programmed ribosomal frameshifting during translation.[4]
If cellular polyamine synthesis is inhibited, cell growth is stopped or severely retarded. The provision of exogenous polyamines restores the growth of these cells. Most eukaryotic cells have a polyamine transporter system on their cell membrane that facilitates the internalization of exogenous polyamines. This system is highly active in rapidly proliferating cells and is the target of some chemotherapeutics currently under development.[5]
Polyamines are also important modulators of a variety of ion channels, including NMDA receptors and AMPA receptors. They block inward-rectifier potassium channels so that the currents of the channels are inwardly rectified, thereby the cellular energy, i.e. K+
ion gradient across the cell membrane, is conserved. In addition, polyamine participate in initiating the expression of SOS response of Colicin E7 operon and down-regulate proteins that are essential for colicin E7 uptake, thus conferring a survival advantage on colicin-producing E. coli under stress conditions.[6]
Polyamines can enhance the permeability of the blood–brain barrier.[7]
They are involved in modulating senescence of organs in plants and are therefore considered as a plant hormone.[8] In addition, they are directly involved in regulation of programmed cell death [9]
Chelating agents
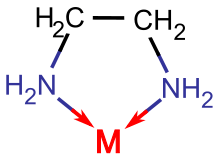
Polyamines are important chelating agents. tetramethylethylenediamine (TMED) is useful for dissolving metal ions in organic solvents. Polyamines like diethylenetriamine (DETA or dien) and triethylenetetramine (TETA or trien) and more powerful chelating agents forming tridentate and tetradentate complexes, respectively. Macrocyclic polyamines like cyclam add cavity selectivity to the chelate effect. The heme group in Hemoglobin is an important example of a macrocyclic ligand containing the polyamine motif.
There are aromatic analogues of the aliphatic linear polyamines such as dipyridine, o-phenanthroline and terpyridine which are also useful chelating agents.
Protonated polyamines, particularly macrocyclic ones, can bind anions. By varying the shape and size of the cavity the protonated polyamine can be engineered to be a specific anion receptor.
Biosynthesis of linear polyamines
Putrescine
Putrescine is synthesized biologically via two different pathways, both starting from arginine.
- In one pathway, arginine is converted into agmatine, with a reaction catalyzed by the enzyme arginine decarboxylase (ADC); then agmatine is transformed into N-carbamoylputrescine by agmatine imino hydroxylase (AIH). Finally, N-carbamoylputrescine is converted into putrescine.[10]
- In the second pathway, arginine is converted into ornithine and then ornithine is converted into putrescine by ornithine decarboxylase (ODC).
Cadaverine

Cadaverine is synthesized from lysine in a one-step reaction with lysine decarboxylase (LDC).
Spermidine and spermine

Spermidine is synthesized from putrescine, using an aminopropyl group from decarboxylated S-adenosyl-L-methionine (SAM). The reaction is catalyzed by spermidine synthase.
Spermine is synthesized from the reaction of spermidine with SAM in the presence of the enzyme spermine synthase.
Thermospermine
Thermospermine is a structural isomer of spermine and a novel type of plant growth regulator. It is produced from spermidine by the action of thermospermine synthase, which is encoded by a gene named ACAULIS5 (ACL5).[11]
Polyamine Analogues
The critical role of polyamines in cell growth has led to the development of a number of agents that interfere with polyamine metabolism. These agents are used in cancer therapy. Polyamine analogues upregulate p53 in a cell leading to restriction of proliferation and apoptosis.[12] It also decreases the expression of estrogen receptor alpha in ER positive breast cancer.[13]
References
- ↑ Pegg, AE; McCann, PP (1982). "Polyamine metabolism and function". American Journal of Physiology. 243: 212–21. PMID 6814260.
- ↑ Seiler, N (1992). "Polyamines". Handbook of Neurochemistry. 1. New York, NY: Plenum Publishing Corp. pp. 223–55.
- ↑ Lawrence, Stephen A. (2004). Amines: synthesis, properties and applications. Cambridge University Press. p. 64. ISBN 978-0-521-78284-5.
- ↑ Rato C; Amirova S.R; Bates D.G; Stansfield I; Wallace H.M (June 2011). "Translational recoding as a feedback controller: systems approaches reveal polyamine-specific effects on the antizyme ribosomal frameshift". Nucleic Acid Res. 39 (11): 4587–4597. doi:10.1093/nar/gkq1349. PMC 3113565
 . PMID 21303766.
. PMID 21303766. - ↑ Wang C, Delcros JG, Cannon L, et al. (November 2003). "Defining the molecular requirements for the selective delivery of polyamine conjugates into cells containing active polyamine transporters". J. Med. Chem. 46 (24): 5129–38. doi:10.1021/jm030223a. PMID 14613316.
- ↑ Yi-Hsuan Pan; Chen-Chung Liao (May 2006). "The critical roles of polyamines regulating ColE7 production and restricting ColE7 uptake of the colicin-producing Escherichia coli". J. Biol. Chem. 281 (19): 13083–13091. doi:10.1074/jbc.M511365200. PMID 16549429.
- ↑ Zhang L, Lee HK, Pruess TH, White HS, Bulaj G (March 2009). "Synthesis and applications of polyamine amino acid residues: improving the bioactivity of an analgesic neuropeptide, neurotensin". J. Med. Chem. 52 (6): 1514–7. doi:10.1021/jm801481y. PMC 2694617
 . PMID 19236044.
. PMID 19236044. - ↑ Pandey S, Ranade SA, Nagar PK, Kumar N (September 2000). "Role of polyamines and ethylene as modulators of plant senescence". J. Biosci. 25 (3): 291–9. doi:10.1007/BF02703938. PMID 11022232.
- ↑ Moschou, PN; Roubelakis-Angelakis, KA (Nov 11, 2013). "Polyamines and programmed cell death.". Journal of Experimental Botany. 65: 1285–1296. doi:10.1093/jxb/ert373. PMID 24218329.
- ↑ Srivenugopal KS, Adiga PR (September 1981). "Enzymic conversion of agmatine to putrescine in Lathyrus sativus seedlings. Purification and properties of a multifunctional enzyme (putrescine synthase).". J. Biol. Chem. 256 (18): 9532–41. PMID 6895223.
- ↑ "Thermospermine is not a minor polyamine in the plant kingdom". Plant Cell Physiology. April 2012. pp. 606–16. doi:10.1093/pcp/pcs019. PMID 22366038. Retrieved 15 October 2015.
- ↑ "Role of p53/p21(Waf1/Cip1) in the regulation of polyamine analogue-induced growth inhibition and cell death in human breast cancer cells". Retrieved 21 November 2012.
- ↑ "Polyamine analogues down-regulate estrogen receptor alpha expression in human breast cancer cells". Retrieved 21 November 2012.
External links
- Polyamines in cell cycle proliferation and cell death
- Ornithine Decarboxylase: Expression and regulation in rat brain and in transgenic mice, 2002, Pekka Kilpelainen, Department of Biochemistry, University of Oulu. Extensive review of literature through 2001 on polyamine structure, properties, metabolism in mammals, and physiological and pathophysiological roles (See article Table of Contents)