Conium maculatum
| Conium maculatum | |
|---|---|
 | |
| Conium maculatum in California | |
| Scientific classification | |
| Kingdom: | Plantae |
| (unranked): | Angiosperms |
| (unranked): | Eudicots |
| (unranked): | Asterids |
| Order: | Apiales |
| Family: | Apiaceae |
| Subfamily: | Apioideae |
| Genus: | Conium |
| Species: | C. maculatum |
| Binomial name | |
| Conium maculatum (L., 1753) | |
| Synonyms[1] | |
|
List
| |

| Wikimedia Commons has media related to Conium maculatum. |
Conium maculatum (hemlock or poison hemlock) is a highly poisonous biennial plant herbaceous flowering plant in the carrot family Apiaceae, native to Europe and North Africa.[2]
Description
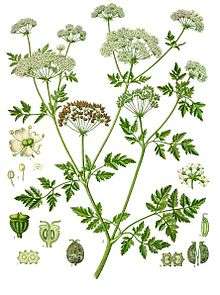
It is a herbaceous biennial plant that grows to 1.5–2.5 m (5–8 ft) tall, with a smooth, green, hollow stem, usually spotted or streaked with red or purple on the lower half of the stem. All parts of the plant are hairless (glabrous). The leaves are two- to four-pinnate, finely divided and lacy, overall triangular in shape, up to 50 cm (20 in) long and 40 cm (16 in) broad. The flowers are small, white, clustered in umbels up to 10–15 cm (4–6 in) across. When crushed, the leaves and root emit a rank, unpleasant odor often compared to that of parsnips. It produces a large number of seeds that allow the plant to form thick stands in modified soils.
Name
Conium maculatum is known by several common names. In addition to the English poison hemlock, the Australian Carrot Fern,[3] and the Irish devil's bread or devil's porridge, poison parsley, spotted corobane, and spotted hemlock are used. The plant should not be confused with the coniferous tree Tsuga, also known by the common name hemlock even though the two plants are quite different. The dried stems are sometimes called kecksies or kex.[4]
Conium comes from the Greek konas (meaning to whirl), in reference to vertigo, one of the symptoms of ingesting the plant.[5]
Distribution
Conium maculatum is native in temperate regions of Europe, West Asia, and North Africa. It has been introduced and naturalised in many other areas, including Asia, North America, Australia, and New Zealand.[6][7][3] It is often found on poorly drained soils, particularly near streams, ditches, and other surface water. It also appears on roadsides, edges of cultivated fields, and waste areas.[6] It is considered an invasive species in 12 U.S. states.[8]
Ecology
Conium maculatum grows in damp areas,[9] but also on drier rough grassland, roadsides, and disturbed ground. It is used as a food plant by the larvae of some Lepidoptera species, including silver-ground carpet. Poison hemlock flourishes in the spring, when most other forage is gone. All plant parts are poisonous, but once the plant is dried, the poison is greatly reduced, although not gone completely.
Biochemistry
-Coniine_Structural_Formula_V.1.svg.png)
Eight piperidinic alkaloids have been identified in C. maculatum. Two of them, gamma-coniceine and coniine, are generally the most abundant, and they account for most of the plant's acute and chronic toxicity. These alkaloids are synthesized by the plant from four acetate units from the metabolic pool, forming a polyketoacid which cyclises through an aminotransferase and forms gamma-coniceine as the parent alkaloid via reduction by a NADPH-dependent reductase.
Poison

Conium contains the piperidine alkaloids coniine, N-methylconiine, conhydrine, pseudoconhydrine, and gamma-coniceine (or g-coniceïne), which is the precursor of the other hemlock alkaloids.[6][10][11]
Coniine has a chemical structure and pharmacological properties similar to nicotine,[6][12] and disrupts the workings of the central nervous system through action on nicotinic acetylcholine receptors. In high enough concentrations, coniine can be dangerous to humans and livestock.[11] Due to high potency, the ingestion of seemingly small doses can easily result in respiratory collapse and death.[13] Coniine causes death by blocking the neuromuscular junction in a manner similar to curare; this results in an ascending muscular paralysis with eventual paralysis of the respiratory muscles which results in death due to lack of oxygen to the heart and brain. Death can be prevented by artificial ventilation until the effects have worn off 48–72 hours later.[6] For an adult, the ingestion of more than 100 mg (0.1 gram) of coniine (about six to eight fresh leaves, or a smaller dose of the seeds or root) may be fatal.[14]
Isolation of the alkaloids
Of the total alkaloids of hemlock isolated by the method of Chemnitius[15] and fractionally distilled, the portion boiling up to 190 °C (374 °F) contains most of the coniine, gamma-coniceine and N-methylconiine, while conhydrine and pseudoconhydrine remain in the higher boiling residues. For the separation of coniine from coniceine, Wolffenstein[16][17] recommends conversion into hydrochlorides. These are dried and extracted with acetone, which dissolves coniceine hydrochloride, leaving the coniine salt, from which the base may then be regenerated. For final purification, the coniine is converted into the D-hydrogen tartrate. It is sometimes necessary to start crystallisation by adding a crystal of the desired salt. Von Braun[18][19] distills the crude mixed alkaloids until the temperature rises to 190 °C (374 °F), benzoylates the distillate, extracts the tertiary bases by shaking an ethereal solution with dilute acid, pours the concentrated ethereal solution into light petroleum to precipitate most of the benzoyl-δ-aminobutyl propyl ketone formed by the action of benzoyl chloride on coniceine, distills the solvent from the filtrate and collects from the residue the fraction boiling at 200–210 °C (392–410 °F)/16 mmHg (2.1 kPa), which is nearly pure benzoylconiine (bp. 203–204 °C (397–399 °F)/16 mmHg). From this a mixture of D- and L-coniines are obtained by hydrolysis, the former predominating.
Uses and effects
Socrates

In ancient Greece, hemlock was used to poison condemned prisoners. The most famous victim of hemlock poisoning is the philosopher Socrates. After being condemned to death for impiety and corrupting the young men of Athens, in 399 BC, Socrates was given a potent infusion of the hemlock plant. Plato described Socrates' death in the Phaedo:[20]
The man...laid his hands on him and after a while examined his feet and legs, then pinched his foot hard and asked if he felt it. He said "No"; then after that, his thighs; and passing upwards in this way he showed us that he was growing cold and rigid. And then again he touched him and said that when it reached his heart, he would be gone. The chill had now reached the region about the groin, and uncovering his face, which had been covered, he said – and these were his last words – "Crito, we owe a cock to Asclepius. Pay it and do not neglect it." "That," said Crito, "shall be done; but see if you have anything else to say." To this question he made no reply, but after a little while he moved; the attendant uncovered him; his eyes were fixed. And Crito when he saw it, closed his mouth and eyes.[21]
Although many have questioned whether this is a factual account, careful attention to Plato's words, modern and ancient medicine, and other ancient Greek sources point to the above account being consistent with Conium poisoning.[22]
Effects on animals
Conium maculatum is poisonous to animals. In a short time, the alkaloids produce a potentially fatal neuromuscular blockage when the respiratory muscles are affected. Acute toxicity, if not lethal, may resolve in the spontaneous recovery of the affected animals provided further exposure is avoided.
It has been observed that poisoned animals tend to return to feed on this plant. Chronic toxicity affects only pregnant animals. When they are poisoned by C. maculatum during the fetus' organ formation period, the offspring is born with malformations, mainly palatoschisis and multiple congenital contractures (MCC; frequently described as arthrogryposis). Chronic toxicity is irreversible and although MCC can be surgically corrected in some cases, most of the malformed animals are lost. Such losses may be underestimated, at least in some regions, because of the difficulty in associating malformations with the much earlier maternal poisoning.
Since no specific antidote is available, prevention is the only way to deal with the production losses caused by the plant. Control with herbicides and grazing with less susceptible animals (such as sheep) have been suggested. C. maculatum alkaloids can enter the human food chain via milk and fowl.[23]
References
- ↑ The Plant List, Conium maculatum L.
- ↑ Altervista Flora Italiana, Cicuta maggiore, Conium maculatum L. includes photos and European distribution map
- 1 2 Atlas of Living Australia, Conium maculatum L., Carrot Fern
- ↑ "Kex". EtymOnline.
- ↑ "Conium maculatum". Northwestern Arizona University. Retrieved 2012-07-06.
- 1 2 3 4 5 Schep, L. J.; Slaughter, R. J.; Beasley, D. M. (2009). "Nicotinic Plant Poisoning". Clinical Toxicology. 47 (8): 771–781. doi:10.1080/15563650903252186. PMID 19778187.
- ↑ Flora of China, 毒参 du shen Conium maculatum Linnaeus, Sp. Pl. 1: 243. 1753.
- ↑ "poison hemlock, spotted hemlock – Conium maculatum". Map, where regarded invasive. National Park Service. Retrieved 2009-01-14.
- ↑ http://www.brc.ac.uk/plantatlas/index.php?q=plant/conium-maculatum
- ↑ Reynolds, T. (June 2005). "Hemlock Alkaloids from Socrates to Poison Aloes". Phytochemistry. 66 (12): 1399–1406. doi:10.1016/j.phytochem.2005.04.039. PMID 15955542.
- 1 2 Vetter, J. (September 2004). "Poison Hemlock (Conium maculatum L.)". Food and Chemical Toxicology. 42 (9): 1373–1382. doi:10.1016/j.fct.2004.04.009. PMID 15234067.
- ↑ Brooks, D. E. (2010-06-28). "Plant Poisoning, Hemlock". MedScape. eMedicine. Retrieved 2012-03-02.
- ↑ Tilford, Gregory L. Edible and Medicinal Plants of the West. ISBN 0-87842-359-1.
- ↑ "Conium maculatum L.". Inchem. IPCS (International Programme on Chemical Safety). Retrieved 2012-07-06.
- ↑ Chemnitius, F. (1928). "Zur Darstellung des Coniins und des Conhydrins". Journal für Praktische Chemie (in German). 118 (1): 25–28. doi:10.1002/prac.19281180105.
- ↑ Wolffenstein, R. (1894). "Ueber Coniin". Berichte der Deutschen Chemischen Gesellschaft (in German). 27 (2): 2615–2621. doi:10.1002/cber.189402702268.
- ↑ Wolffenstein, R. (1895). "Ueber Coniumalkaloïde". Berichte der Deutschen Chemischen Gesellschaft (in German). 28 (1): 302–305. doi:10.1002/cber.18950280171.
- ↑ von Braun, J. (1905). "Ueber die Trennung der Coniumalkaloïde". Berichte der Deutschen Chemischen Gesellschaft (in German). 38 (3): 3108–3112. doi:10.1002/cber.190503803125.
- ↑ von Braun, J. (1917). "Notiz über das N-Methyl-coniin". Berichte der Deutschen Chemischen Gesellschaft (in German). 50 (2): 1477. doi:10.1002/cber.19170500246.
- ↑ "Plato, Euthyphro, Apology, Crito, Phaedo". Retrieved 2012-07-06.
- ↑ Plato, Phaedo 117e–118a. trans. Loeb Classical Library (1990 ed.). Cambridge, MA: Harvard University Press. pp. 401–3.
- ↑ Bloch, E. (March 2001). "Hemlock Poisoning and the Death of Socrates: Did Plato Tell the Truth?". Journal of the International Plato Society (1).—A version of this article was also printed in Bloch, E. (2001). Brickhouse, Thomas C.; Smith, Nicholas D., eds. The Trial and Execution of Socrates: Sources and Controversies. New York: Oxford University Press. ISBN 978-0-19-511980-0.
- ↑ "Conium maculatum, Poison Hemlock – Element Stewardship Abstract" (PDF). Conserve Online. Retrieved 2012-03-02.
External links
| Wikisource has the text of the 1905 New International Encyclopedia article Hemlock. |
- "Conium". USDA Agricultural Research Service – Germplasm Resources Information Network.
- "Conium". Flora Europaea. Royal Botanic Garden Edinburgh.