Phosphonate
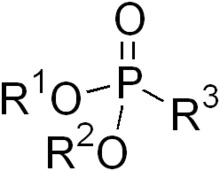
Phosphonates and phosphonic acids are organophosphorus compounds containing C−PO(OH)2 or C−PO(OR)2 groups (where R = alkyl, aryl). Phosphonic acids and phosphonate salts are typically white, nonvolatile solids that are poorly soluble in organic solvents, but soluble in water and common alcohols. Many commercially important compounds are phosphonates, including glyphosate, the herbicide "Roundup", and ethephon, a widely used plant growth regulator. Bisphosphonates are popular drugs for treatment of osteoporosis.[1]

Basic properties
Phosphonates feature tetrahedral phosphorus centers. They are structurally closely related to (and often prepared from) phosphorous acid.[2]

Phosphonate salts are the result of deprotonation of phosphonic acids, which are diprotic acids:
- RPO(OH)2 + NaOH → H2O + RPO(OH)(ONa) (monosodium phosphonate)
- RPO(OH)(ONa) + NaOH → H2O + RPO(ONa)2 (disodium phosphonate)
Phosphonate esters are the result of condensation of phosphonic acids with alcohols.
Production
Several methods exist for the preparation of phosphonic acids and their salts.
From phosphonic acid
Most processes begin with phosphorous acid (aka phosphonic acid, H3PO3), exploiting its reactive P−H bond.[1][2]
Phosphonic acid can be alkylated under Mannich conditions to give aminomethylated phosphonates, which are useful as complexants. One example is the industrial preparation of nitrilotris(methylenephosphonic acid):
- NH3 + 3 H3PO3 + 3 CH2O → N(CH2PO3H2)3 + 3 H2O
Phosphonic acid also can be alkylated with acrylic acid derivatives to afford carboxyl functionalized phosphonic acids. This reaction is a variant of the Michael addition:
- CH2=CHCO2R + 3 H3PO3 → (HO)2P(O)CH2CH2CO2R
Michaelis-Arbuzov reaction
Phosphonic esters are prepared using the Michaelis–Arbuzov reaction. For example, methyl iodide catalyses the conversion of trimethylphosphite to the phosphonate ester dimethyl methylphosphonate:
- P(OMe)3 → MePO(OMe)2
These esters can be hydrolysed to the acid (Me = methyl):
- MePO(OMe)2 + H2O → MePO(OH)2 + 2 MeOH
In the Michaelis–Becker reaction, a hydrogen phosphonate diester is first deprotonated and the resulting anion is alkylated.
From phosphorus trichloride
Vinylphosphonic acid can be prepared by the reaction of PCl3 and acetaldehyde:
- PCl3 + CH3CHO → CH3CH(O−)PCl+
3
This adduct reacts with acetic acid:
- CH3CH(O−)PCl+
3 + 2 CH3CO2H → CH3CH(Cl)PO(OH)2 + 2 CH3COCl
This chloride undergoes dehydrochlorination to afford the target:
- CH3CH(Cl)PO(OH)2 → CH2=CHPO(OH)2 + HCl
Bisphosphonates
Bisphosphonates were first synthesized in 1897 by Von Baeyer and Hofmann. An example of such a bisphosphonate is HEDP (etidronic acid or Didronel), that is 1-hydroxyethane-1,1-diphosphonic acid, which is prepared from phosphorous acid and acetic anhydride:[1]
- 2 H3PO3 + (CH3CO)2O → CH3C(OH)(PO3H2)2 + CH3CO2H
Occurrence in nature

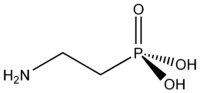
Phosphonates are one of the three sources of phosphate intake in biological cells. The other two are inorganic phosphate and organophosphates.
The naturally-occurring phosphonate 2-aminoethylphosphonic acid was first identified in 1959 in plants and many animals, where it is localized in membranes. Phosphonates are quite common among different organisms, from prokaryotes to eubacteria and fungi, mollusks, insects and others. They were first reported in natural soils by Newman and Tate (1980). The biological role of the natural phosphonates is still poorly understood. Bis- or polyphosphonates have not been found to occur naturally.
Uses
In 1998 the consumption of phosphonates was 56,000 tons worldwide – 40,000 tons in the US, 15,000 tons in Europe and less than 800 tons in Japan. The demand of phosphonates grows steadily at 3% annually.
Metal chelants
Since the work of Schwarzenbach in 1949, phosphonic acids are known as effective chelating agents. The introduction of an amine group into the molecule to obtain −NH2−C−PO(OH)2 increases the metal binding abilities of the phosphonate. Examples for such compounds are NTMP, EDTMP and DTPMP. These phosphonates are the structural analogues to the well-known aminopolycarboxylate such as EDTA. The stability of the metal complexes increases with increasing number of phosphonic acid groups. Phosphonates are highly water-soluble while the phosphonic acids are only sparingly so.
Phosphonates are effective chelating agents. That is, they bind tightly to di- and trivalent metal ions, which is useful in water softening. In this way, they prevent formation of insoluble precipitates (scale). The binding of these ligands also suppresses the catalytic properties of metal ions. They are stable under harsh conditions. For these reasons, an important industrial use of phosphonates is in cooling waters, desalination systems, and in oil fields to inhibit scale formation. Phosphonates are also regularly used in reverse osmosis systems as anti-scalants. Phosphonates in cooling water systems also serve to control corrosion of iron and steel. In pulp and paper manufacturing and in textile industry they serve as "peroxide bleach stabilizers", by chelating metals that could inactivate the peroxide. In detergents they are used as a combination of chelating agent, scale inhibitor, and bleach stabilizer. Phosphonates are also increasingly used in medicine to treat disorders associated with bone formation and calcium metabolism. Furthermore, they serve as carriers for radionuclides in bone cancer treatments (see samarium-153-ethylene diamine tetramethylene phosphonate).
Medicine
In medicine, phosphonates and bisphosphonates are commonly used as inhibitors of enzymes which utilize phosphates and diphosphates as substrates. Most notably, these enzymes include those that produce the intermediates of cholesterol biosynthesis.[3]
Niche uses
In organic synthesis, phosphonates are used in the Horner–Wadsworth–Emmons reaction.
In conjunction with organosilicates, phosphonates are also used to treat "sudden oak death", which is caused by the fungus-like eukaryote Phytophthora ramorum.
Toxicology
The toxicity of phosphonates to aquatic organisms is low. Reported values for 48-hour LC50 values for fish are between 0.1 and 1.1 mM. Also the bioconcentration factor for fish is very low.
Biodegradation
In nature bacteria play a major role in the degradation of phosphonates.[4] Due to the presence of natural phosphonates in the environment, bacteria have evolved the ability to metabolize phosphonates as nutrient sources. Some bacteria use phosphonates as a phosphorus source for growth. Aminophosphonates can also be used as sole nitrogen source by some bacteria. The polyphosphonates used in industry differ greatly from natural phosphonates such as 2-aminoethylphosphonic acid, because they are much larger, carry a high negative charge and are complexed with metals. Biodegradation tests with sludge from municipal sewage treatment plants with HEDP and NTMP showed no indication for any degradation. An investigation of HEDP, NTMP, EDTMP and DTPMP in standard biodegradation tests also failed to identify any biodegradation. It was noted, however, that in some tests due to the high sludge to phosphonate ratio, removal of the test substance from solution observed as loss of DOC was observed. This factor was attributed to adsorption rather than biodegradation. However, bacterial strains capable of degrading aminopolyphosphonates and HEDP under P-limited conditions have been isolated from soils, lakes, wastewater, activated sludge and compost.
"No biodegradation of phosphonates during water treatment is observed but photodegradation of the Fe(III)-complexes is rapid. Aminopolyphosphonates are also rapidly oxidized in the presence of Mn(II) and oxygen and stable breakdown products are formed that have been detected in wastewater. The lack of information about phosphonates in the environment is linked to analytical problems of their determination at trace concentrations in natural waters. Phosphonates are present mainly as Ca and Mg-complexes in natural waters and therefore do not affect metal speciation or transport."[5] Phosphonates interact strongly with some surfaces, which results in a significant removal in technical and natural systems.
Phosphonate compounds
- AMPA: Aminomethylphosphonic acid, degradation product of glyphosate
- Vinylphosphonic acid: monomer
- DMMP: Dimethyl methylphosphonate, one of the simplest phosphonate diesters
- HEDP: 1-Hydroxyethylidene-1,1-diphosphonic acid, used in detergents, water treatment, cosmetics and pharmaceuticals
- ATMP: Aminotris(methylenephosphonic acid), chelating agent
- EDTMP: Ethylenediaminetetra(methylenephosphonic acid), chelating agent
- TDTMP: Tetramethylenediaminetetra(methylenephosphonic acid), chelating agent
- HDTMP: Hexamethylenediaminetetra(methylenephosphonic acid), chelating agent
- DTPMP: Diethylenetriaminepenta(methylenephosphonic acid), chelating agent
- PBTC: Phosphonobutanetricarboxylic acid
- PMIDA: N-(phosphonomethyl)iminodiacetic acid
- CEPA: 2-carboxyethyl phosphonic acid
- HPAA: 2-Hydroxyphosphonocarboxylic acid
- AMP: Aminotris(methylenephosphonic acid)
- BPMG: N,N-Bis(phosphonomethyl)glycine
- Glyphosate, a common agricultural herbicide
- Foscarnet, for treatment of herpes
- Perzinfotel, for treatment of stroke
See also
- Organophosphorus compounds
- Phosphine oxide – OPR3
- Phosphinite – P(OR)R2
- Phosphonite – P(OR)2R
- Phosphite – P(OR)3
- Phosphinate – OP(OR)R2
- Phosphate – OP(OR)3
References
- 1 2 3 Svara, J.; Weferling, N.; Hofmann, T. "Phosphorus Compounds, Organic," in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2008. doi:10.1002/14356007.a19_545.pub2.
- 1 2 Modern Phosphonate Chemistry by Philippe Savignac and Bogdan Iorga, CRC Press, Boca Raton, FL, 2003. ISBN 0-8493-1099-7.
- ↑ Wiemer, AJ; Hohl, RJ; Wiemer, DF (June 2009). "The intermediate enzymes of isoprenoid metabolism as anticancer targets.". Anti-cancer agents in medicinal chemistry. 9 (5): 526–42. doi:10.2174/187152009788451860. PMID 19519294.
- ↑ Huang J, Su Z, Xu Y (November 2005). "The evolution of microbial phosphonate degradative pathways". Journal of Molecular Evolution. 61 (5): 682–90. doi:10.1007/s00239-004-0349-4. PMID 16245012.
- ↑ Nowack, Bernd "Environmental chemistry of phosphonates" Water Research 2003, vol. 37, p 2533–2546. doi:10.1016/S0043-1354(03)00079-4
Additional reading
- Newman, R.H., and K.R. Tate. 1980. "Soil characterized by 31P nuclear magnetic resonance" Communications in Soil Science and Plant Analysis, 11, 835–842.
- Abhimanyu S. Paraskar & Arumugam Sudalai (2006). "A novel Cu(OTf)2 mediated three component high yield synthesis of α-aminophosphonates" (PDF). Arkivoc (1838EP): 183–9.
- Singh R, Nolan SP (November 2005). "Synthesis of phosphorus esters by transesterification mediated by N-heterocyclic carbenes (NHCs)". Chemical Communications (43): 5456–8. doi:10.1039/b509783e. PMID 16261245.