Phosphoenolpyruvate carboxylase
| Phosphoenolpyruvate carboxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 4.1.1.31 | ||||||||
| CAS number | 9067-77-0 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| Phosphoenolpyruvate carboxylase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | PEPcase | ||||||||
| Pfam | PF00311 | ||||||||
| InterPro | IPR001449 | ||||||||
| PROSITE | PDOC00330 | ||||||||
| SCOP | 1fiy | ||||||||
| SUPERFAMILY | 1fiy | ||||||||
| |||||||||
Phosphoenolpyruvate carboxylase (also known as PEP carboxylase, PEPCase, or PEPC; EC 4.1.1.31, PDB ID: 3ZGE) is an enzyme in the family of carboxy-lyases found in plants and some bacteria that catalyzes the addition of bicarbonate (HCO3−) to phosphoenolpyruvate (PEP) to form the four-carbon compound oxaloacetate and inorganic phosphate:[1]
- PEP + HCO3− → oxaloacetate + Pi
This reaction is used for carbon fixation in CAM (crassulacean acid metabolism) and C4 organisms, as well as to regulate flux through the citric acid cycle (also known as Krebs or TCA cycle) in bacteria and plants. The enzyme structure and its two step catalytic, irreversible mechanism have been well studied. PEP carboxylase is highly regulated, both by phosphorylation and allostery.
Enzyme structure
The PEP carboxylase enzyme is present in plants and some types of bacteria, but not in fungi or animals (including humans).[2] The genes vary between organisms, but are strictly conserved around the active and allosteric sites discussed in the mechanism and regulation sections. Tertiary structure of the enzyme is also conserved.[3]
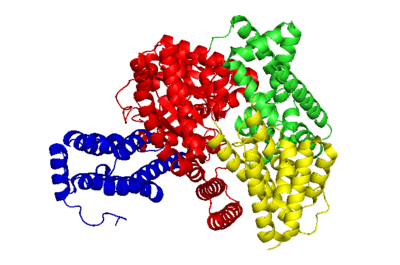
The crystal structure of PEP carboxylase in multiple organisms, including Zea mays (maize), and Escherichia coli has been determined.[3] The overall enzyme exists as a dimer-of-dimers: two identical subunits closely interact to form a dimer through salt bridges between arginine (R438 - exact positions may vary depending on the origin of the gene) and glutamic acid (E433) residues.[4] This dimer assembles (more loosely) with another of its kind to form the four subunit complex. The monomer subunits are mainly composed of alpha helices (65%),[1] and have a mass of 106kDa each.[5] The sequence length is about 966 amino acids.[6] See figure 1 for a PyMOL generated structure of the enzyme’s single subunit from the organism Flaveria trinervia.
The enzyme active site is not completely characterized. It includes a conserved aspartic acid (D564) and a glutamic acid (E566) residue that non-covalently bind a divalent metal cofactor ion through the carboxylic acid functional groups.[1] This metal ion can be magnesium, manganese or cobalt depending on the organism,[1][2] and its role is to coordinate the phosphoenolpyruvate molecule as well as the reaction intermediates. A histidine (H138) residue at the active site is believed to facilitate proton transfer during the catalytic mechanism.[1][4]
Enzyme mechanism
The mechanism of PEP carboxylase has been well studied. The enzymatic mechanism of forming oxaloacetate is very exothermic and thereby irreversible; the biological Gibbs free energy change (△G°’) is -30kJmol−1.[1] The substrates and cofactor bind in the following order: metal cofactor (either Co2+, Mg2+, or Mn2+), PEP, bicarbonate (HCO3−).[1][2] The mechanism proceeds in two major steps, as described below and shown in figure 2:
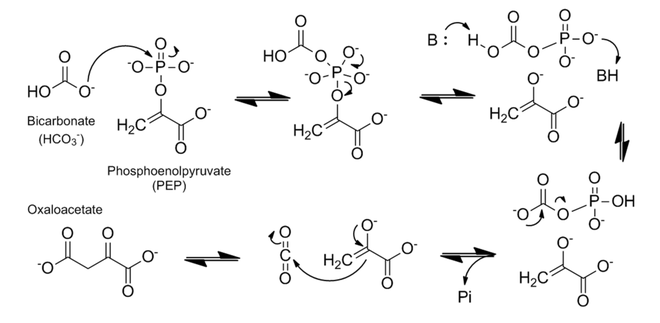
1. The bicarbonate acts as a nucleophile to attack the phosphate group in PEP. This results in the splitting of PEP into a carboxyphosphate and the (very reactive) enolate form of pyruvate.
2. Proton transfer takes place at the carboxyphosphate. This is most likely modulated by a histidine (H138) residue that first deprotonates the carboxy side, and then, as an acid, protonates the phosphate part.[1] The carboxyphosphate then exothermically decomposes into carbon dioxide and inorganic phosphate, at this point making this an irreversible reaction. Finally, after the decomposition, the carbon dioxide is attacked by the enolate to form oxaloacetate.[1][2][7]
The metal cofactor is necessary to coordinate the enolate and carbon dioxide intermediates; the CO2 molecule is only lost 3% of the time.[2] The active site is hydrophobic to exclude water, since the carboxyphosphate intermediate is susceptible to hydrolysis.[1]
Biological function
The three most important roles that PEP carboxylase plays in plants and bacteria metabolism are in the C4 cycle, the CAM cycle, and the citric acid cycle biosynthesis flux.
The primary mechanism of carbon dioxide assimilation in plants is through the enzyme ribulose-1,5-bisphosphate carboxylase/oxygenase (also known as RuBisCO), that adds CO2 to ribulose-1,5-bisphosphate (a 5 carbon sugar), to form two molecules of 3-phosphoglycerate (2x3 carbon sugars). However, at higher temperatures and lower CO2 concentrations, RuBisCO adds oxygen instead of carbon dioxide, to form the unusable product glycolate in a process called photorespiration. To prevent this wasteful process, plants increase the local CO2 concentration in a process called the C4 cycle.[3][8] PEP carboxylase plays the key role of binding CO2 in the form of bicarbonate with PEP to create oxaloacetate in the mesophyll tissue. This is then converted back to pyruvate (through a malate intermediate), to release the CO2 in the deeper layer of bundle sheath cells for carbon fixation by RuBisCO and the Calvin cycle. Pyruvate is converted back to PEP in the mesophyll cells, and the cycle begins again, thus actively pumping CO2.[2][9][10]
The second important and very similar biological significance of PEP carboxylase is in the CAM cycle. This cycle is common in organisms living in arid habitats. Plants cannot afford to open stomata during the day to take in CO2, as they would lose too much water by transpiration. Instead, stomata open at night, when water evaporation is minimal, and take in CO2 by fixing with PEP to form oxaloacetate though PEP carboxylase. Oxaloacetate is converted to malate by malate dehydrogenase, and stored for use during the day when the light dependent reaction generates energy (mainly in the form of ATP) and reducing equivalents such as NADPH to run the Calvin cycle.[2][3][10]
Third, PEP carboxylase is significant in non-photosynthetic metabolic pathways. Figure 3 shows this metabolic flow (and its regulation). Similar to pyruvate carboxylase, PEP carboxylase replenishes oxaloacetate in the citric acid cycle. At the end of glycolysis, PEP is converted to pyruvate, which is converted to acetyl-coenzyme-A (acetyl-CoA), which enters the citric acid cycle by reacting with oxaloacetate to form citrate. To increase flux through the cycle, some of the PEP is converted to oxaloacetate by PEP carboxylase. Since the citric acid cycle intermediates provide a hub for metabolism, increasing flux is important for the biosynthesis of many molecules, such as for example amino acids.[11]
Enzyme regulation
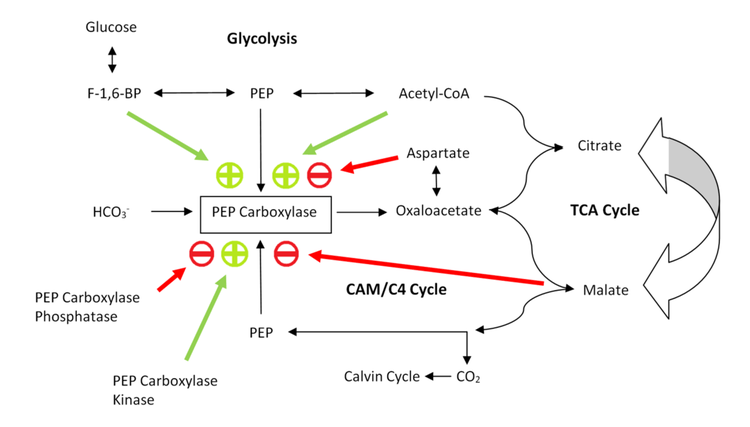
PEP carboxylase is mainly subject to two levels of regulation: phosphorylation and allostery. Figure 3 shows a schematic of the regulatory mechanism.
Phosphorylation by phosphoenolpyruvate carboxylase kinase turns the enzyme on, whereas phosphoenolpyruvate carboxylase phosphatase turns it back off. Both kinase and phosphate are regulated by transcription. It is further believed that malate acts as a feedback inhibitor of kinase expression levels, and as an activator for phosphatase expression (transcription).[12] Since oxaloacetate is converted to malate in CAM and C4 organisms, high concentrations of malate activate phosphatase expression - the phosphatase subsequently de-phosphorylates and thus de-actives PEP carboxylase, leading to no further accumulation of oxaloacetate and thus no further conversion of oxaloacetate to malate. Hence malate production is down-regulated.[1][12]
The main allosteric inhibitors of PEP carboxylase are the carboxylic acids malate (weak) and aspartate (strong).[5][12] Since malate is formed in the next step of the CAM and C4 cycles after PEP carboxylase catalyses the condensation of CO2 and PEP to oxaloacetate, this works as a feedback inhibition pathway. Oxaloacetate and aspartate are easily inter-convertible through a transaminase mechanism; thus high concentrations of aspartate are also a pathway of feedback inhibition of PEP carboxylase.
The main allosteric activators of PEP carboxylase are acetyl-CoA[13] and fructose-1,6-bisphosphate (F-1,6-BP).[1][13] Both molecules are indicators of increased glycolysis levels, and thus positive feed-forward effectors of PEP carboxylase. They signal the need to produce oxaloacetate to allow more flux through the citric acid cycle. Additionally, increased glycolysis means a higher supply of PEP is available, and thus more storage capacity for binding CO2 in transport to the Calvin cycle. It is also noteworthy that the negative effectors aspartate competes with the positive effector acetyl-CoA, suggesting that they share an allosteric binding site.[14]
Studies have shown that energy equivalents such as AMP, ADP and ATP have no significant effect on PEP carboxylase.[15]
The magnitudes of the allosteric effects of these different molecules on PEP carboxylase activity depend on individual organisms.[16]
References
- 1 2 3 4 5 6 7 8 9 10 11 12 Kai, Yasushi; Matsumura, Hiroyoshi; Izui, Katsura (2003). "Phosphoenolpyruvate carboxylase: three-dimensional structure and molecular mechanisms". Archives of Biochemistry and Biophysics. 414 (2): 170–179. doi:10.1016/S0003-9861(03)00170-X. ISSN 0003-9861. PMID 12781768.
- 1 2 3 4 5 6 7 Chollet, Raymond; Vidal, Jean; O'Leary, Marion H. (1996). "PHOSPHOENOLPYRUVATE CARBOXYLASE: A Ubiquitous, Highly Regulated Enzyme in Plants". Annual Review of Plant Physiology and Plant Molecular Biology. 47 (1): 273–298. doi:10.1146/annurev.arplant.47.1.273. ISSN 1040-2519.
- 1 2 3 4 Paulus, Judith Katharina; Schlieper, Daniel; Groth, Georg (2013). "Greater efficiency of photosynthetic carbon fixation due to single amino-acid substitution". Nature Communications. 4 (2): 1518. doi:10.1038/ncomms2504. ISSN 2041-1723. PMC 3586729
 . PMID 23443546.
. PMID 23443546. - 1 2 Kai, Y.; Matsumura, H.; Inoue, T.; Terada, K.; Nagara, Y.; Yoshinaga, T.; Kihara, A.; Tsumura, K.; Izui, K. (1999). "Three-dimensional structure of phosphoenolpyruvate carboxylase: A proposed mechanism for allosteric inhibition". Proceedings of the National Academy of Sciences. 96 (3): 823–828. doi:10.1073/pnas.96.3.823. ISSN 0027-8424.
- 1 2 Gonzalez, Daniel H.; Iglesias, Alberto A.; Andreo, Carlos S. (1986). "Active-site-directed inhibition of phosphoenolpyruvate carboxylase from maize leaves by bromopyruvate". Archives of Biochemistry and Biophysics. 245 (1): 179–186. doi:10.1016/0003-9861(86)90203-1. ISSN 0003-9861. PMID 3947097.
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=3ZGE
- ↑ Fujita, Nobuyuki; Izui, Katsura; Nishino, Tokuzo; Katsuki, Hirohiko (1984). "Reaction mechanism of phosphoenolpyruvate carboxylase. Bicarbonate-dependent dephosphorylation of phosphoenol-.alpha.-ketobutyrate". Biochemistry. 23 (8): 1774–1779. doi:10.1021/bi00303a029. ISSN 0006-2960. PMID 6326809.
- ↑ Leegood, Richard C (2007). "A welcome diversion from photorespiration". Nature Biotechnology. 25 (5): 539–540. doi:10.1038/nbt0507-539. ISSN 1087-0156. PMID 17483837.
- ↑ Hatch, Marshall D. (2002). "C(4) photosynthesis: discovery and resolution". Photosynthesis Research. 73 (1/3): 251–256. doi:10.1023/A:1020471718805. ISSN 0166-8595. PMID 16245128.
- 1 2 Keeley, Jon E.; Rundel, Philip W. (2003). "Evolution of CAM and C4Carbon‐Concentrating Mechanisms". International Journal of Plant Sciences. 164 (S3): S55–S77. doi:10.1086/374192. ISSN 1058-5893.
- ↑ Cousins, A. B.; Baroli, I.; Badger, M. R.; Ivakov, A.; Lea, P. J.; Leegood, R. C.; von Caemmerer, S. (2007). "The Role of Phosphoenolpyruvate Carboxylase during C4 Photosynthetic Isotope Exchange and Stomatal Conductance". Plant Physiology. 145 (3): 1006–1017. doi:10.1104/pp.107.103390. ISSN 0032-0889. PMC 2048775
 . PMID 17827274.
. PMID 17827274. - 1 2 3 Nimmo, Hugh G (2000). "The regulation of phosphoenolpyruvate carboxylase in CAM plants". Trends in Plant Science. 5 (2): 75–80. doi:10.1016/S1360-1385(99)01543-5. ISSN 1360-1385. PMID 10664617.
- 1 2 Morikawa M, Izui K, Taguchi M, Katsuki H (February 1980). "Regulation of Escherichia coli phosphoenolpyruvate carboxylase by multiple effectors in vivo. Estimation of the activities in the cells grown on various compounds". J. Biochem. 87 (2): 441–9. PMID 6987214.
- ↑ Smith, Thomas E. (1970). "Escherichia coli phosphoenolpyruvate carboxylase: Competitive regulation by acetyl-coenzyme A and aspartate". Archives of Biochemistry and Biophysics. 137 (2): 512–522. doi:10.1016/0003-9861(70)90469-8. ISSN 0003-9861. PMID 4909168.
- ↑ Coombs, J.; Maw, Susan L.; Baldry, C. W. (1974). "Metabolic regulation in C4 photosynthesis: PEP-carboxylase and energy charge". Planta. 117 (4): 279–292. doi:10.1007/BF00388023. ISSN 0032-0935. PMID 24458459.
- ↑ Schuller, K. A.; Plaxton, W. C.; Turpin, D. H. (1990). "Regulation of Phosphoenolpyruvate Carboxylase from the Green Alga Selenastrum minutum: Properties Associated with Replenishment of Tricarboxylic Acid Cycle Intermediates during Ammonium Assimilation". Plant Physiology. 93 (4): 1303–1311. doi:10.1104/pp.93.4.1303. ISSN 0032-0889. PMC 1062672
 . PMID 16667617.
. PMID 16667617.