Pertussis toxin
| Pertussis toxin, subunit 1 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
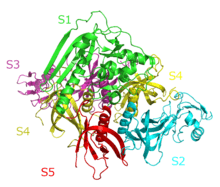 The Crystal Structure of Pertussis Toxin,[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Pertussis_S1 | ||||||||
| Pfam | PF02917 | ||||||||
| InterPro | IPR003898 | ||||||||
| SCOP | 1bcp | ||||||||
| SUPERFAMILY | 1bcp | ||||||||
| |||||||||
| Pertussis toxin, subunit 2 and 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Pertussis_S2S3 | ||||||||
| Pfam | PF02918 | ||||||||
| InterPro | IPR003899 | ||||||||
| SCOP | 1bcp | ||||||||
| SUPERFAMILY | 1bcp | ||||||||
| |||||||||
| Pertussis toxin, subunit 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Pertus-S4-tox | ||||||||
| Pfam | PF09275 | ||||||||
| InterPro | IPR015355 | ||||||||
| SCOP | 1prt | ||||||||
| SUPERFAMILY | 1prt | ||||||||
| |||||||||
| Pertussis toxin, subunit 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | Pertus-S5-tox | ||||||||
| Pfam | PF09276 | ||||||||
| InterPro | IPR015356 | ||||||||
| SCOP | 1prt | ||||||||
| SUPERFAMILY | 1prt | ||||||||
| |||||||||
Pertussis toxin (PT) is a protein-based AB5-type exotoxin produced by the bacterium Bordetella pertussis,[2] which causes whooping cough. PT is involved in the colonization of the respiratory tract and the establishment of infection.[3] Research suggests PT may have a therapeutic role in treating a number of common human ailments, including hypertension,[4] viral inhibition,[5] and autoimmune inhibition.[6][7][8]
History
PT clearly plays a central role in the pathogenesis of pertussis although this was discovered only in the early 1980s. The appearance of pertussis is quite recent, compared with other epidemic infectious diseases. The earliest mention of pertussis, or whooping cough, is of an outbreak in Paris in 1414. This was published in Moulton’s The Mirror of Health, in 1640. Another epidemic of pertussis took place in Paris in 1578 and was described by a contemporary observer, Guillaume de Baillou. Pertussis was well known throughout Europe by the middle of the 18th century. Jules Bordet and Octave Gengou described in 1900 the finding of a new “ovoid bacillus” in the sputum of a 6-month-old infant with whooping cough. They were also the first to cultivate Bordetella pertussis at the Pasteur Institute in Brussels in 1906.[9]
One difference between the different species of Bordetella is that B. pertussis produces PT and the other species do not. Bordetella parapertussis shows the most similarity to B. pertussis and was therefore used for research determining the role of PT in causing the typical symptoms of whooping cough. Rat studies showed the development of paroxysmal coughing, a characteristic for whooping cough, occurred in rats infected with B. pertussis. Rats infected with B. parapertussis or a PT-deficient mutant of B. pertussis did not show this symptom; neither of these two strains produced PT.[10]
Structure
A large group of bacterial exotoxins are referred to as "A/B toxins", in essence because they are formed from two subunits.[11] The "A" subunit possesses enzyme activity, and is transferred to the host cell following a conformational change in the membrane-bound transport "B" subunit.[11] Pertussis toxin is an exotoxin with six subunits (named S1 through S5—each complex contains two copies of S4).[12][13] The subunits are arranged in A-B structure: the A component is enzymatically active and is formed from the S1 subunit, while the B component is the receptor-binding portion and is made up of subunits S2–S5.[13] The subunits are encoded by ptx genes encoded on a large PT operon that also includes additional genes that encode Ptl proteins. Together, these proteins form the PT secretion complex.[14]
Mechanism of pathogenesis
PT is released from B. pertussis in an inactive form. Following PT binding to a cell membrane receptor, it is taken up in an endosome, after which it undergoes retrograde transport to the trans-Golgi network and endoplasmic reticulum.[15] At some point during this transport, the A subunit (or protomer) becomes activated, perhaps through the action of glutathione and ATP.[12][16] PT catalyzes the ADP-ribosylation of the αi subunits of the heterotrimeric G protein. This prevents the G proteins from interacting with G protein-coupled receptors on the cell membrane, thus interfering with intracellular communication.[17] The Gi subunits remain locked in their GDP-bound, inactive state, thus unable to inhibit adenylate cyclase activity, leading to increased cellular concentrations of cAMP.
Increased intracellular cAMP affects normal biological signaling. The toxin causes several systemic effects, among which is an increased release of insulin, causing hypoglycemia. Whether the effects of pertussis toxin are responsible for the paroxysmal cough remains unknown.[18]
As a result of this unique mechanism, PT has also become widely used as a biochemical tool to ADP-ribosylate GTP-binding proteins in the study of signal transduction.[1] It has also become an essential component of new acellular vaccines.[1]
Effects on the immune system
PT has been shown to affect the innate immune response. It inhibits the early recruitment of neutrophils and macrophages, and interferes with the early chemokine production and the inhibition of the neutrophil chemotaxis.[19] Chemokines are signaling molecules produced by infected cells and attract neutrophils and macrophages. Neutrophil chemotaxis is thought to be disrupted by inhibiting G-protein-coupled chemokine receptors by the ADP-ribosylation of Gi proteins.[20]
Because of the disrupted signaling pathways, synthesis of chemokines will be affected. This will prevent the infected cell from producing them and thereby inhibiting recruitment of neutrophils. Under normal circumstances, alveolar macrophages and other lung cells produce a variety of chemokines. PT has been found to inhibit the early transcription of keratinocyte-derived chemokine, macrophage inflammatory protein 2 and LPS-induced CXC chemokine.[20] Eventually, PT causes lymphocytosis, one of the systemic manifestations of whooping cough.[21]
PT, a decisive virulence determinant of B. pertussis, is able to cross the blood–brain barrier by increasing its permeability.[22] As a result, PT can cause severe neurological complications; however, recently it has been found that the medicinal usage of Pertussis toxin can promote the development of regulatory T cells and prevent central nervous system autoimmune disease, such as multiple sclerosis.[23]
Metabolism
PT is known to dissociate into two parts in the endoplasmic reticulum (ER): the enzymatically active A subunit (S1) and the cell-binding B subunit. The two subunits are separated by proteolic cleavage. The B subunit will undergo ubiquitin-dependent degradation by the 26S proteasome. However, the A subunit lacks lysine residues, which are essential for ubiquitin-dependent degradation. Therefore, PT subunit A will not be metabolized like most other proteins.[24]
PT is heat-stable and protease-resistant, but once the A and B are separated, these properties change. The B subunit will stay heat-stable at temperatures up to 60 °C, but it is susceptible to protein degradation. PT subunit A, on the other hand, is less susceptible to ubiquitin-dependent degradation, but is unstable at temperature of 37 °C. This facilitates unfolding of the protein in the ER and tricks the cell into transporting the A subunit to the cytosol, where normally unfolded proteins will be marked for degradation. So, the unfolded conformation will stimulate the ERAD-mediated translocation of PT A into the cytosol. Once in the cytosol, it can bind to NAD and form a stable, folded protein again. Being thermally unstable is also the Achilles heel of PT subunit A. As always, there is an equilibrium between the folded and unfolded states. When the protein is unfolded, it is susceptible to degradation by the 20S proteasome, which can degrade only unfolded proteins.[24]
See also
References
- 1 2 3 Stein PE, Boodhoo A, Armstrong GD, Cockle SA, Klein MH, Read RJ (January 1994). "The crystal structure of pertussis toxin". Structure. 2 (1): 45–57. doi:10.1016/S0969-2126(00)00007-1. PMID 8075982.
- ↑ Ryan KJ; Ray CG (editors) (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 0-8385-8529-9.
- ↑ Carbonetti NH, Artamonova GV, Mays RM, Worthington ZE (November 2003). "Pertussis Toxin Plays an Early Role in Respiratory Tract Colonization by Bordetella pertussis". Infect. Immun. 71 (11): 6358–66. doi:10.1128/IAI.71.11.6358-6366.2003. PMC 219603
 . PMID 14573656.
. PMID 14573656. - ↑ Kost C, Herzer W, Li P, Jackson E (1999). "Pertussis toxin-sensitive G-proteins and regulation of blood pressure in the spontaneously hypertensive rat". Clin Exp Pharmacol Physiol. 26 (5–6): 449–55. doi:10.1046/j.1440-1681.1999.03058.x. PMID 10386237.
- ↑ Alfano M, Pushkarsky T, Poli G, Bukrinsky M (2000). "The B-Oligomer of Pertussis Toxin Inhibits Human Immunodeficiency Virus Type 1 Replication at Multiple Stages". J Virol. 74 (18): 8767–70. doi:10.1128/JVI.74.18.8767-8770.2000. PMC 116391
 . PMID 10954581.
. PMID 10954581. - ↑ Bagley K, Abdelwahab S, Tuskan R, Fouts T, Lewis G (2002). "Pertussis toxin and the adenylate cyclase toxin from Bordetella pertussis activate human monocyte-derived dendritic cells and dominantly inhibit cytokine production through a cAMP-dependent pathway". J Leukoc Biol. 72 (5): 962–9. PMID 12429718.
- ↑ Locht C, Keith JM (1986). "Pertussis toxin gene: nucleotide sequence and genetic organization". Science. 232 (4755): 1258–1264. doi:10.1126/science.3704651. PMID 3704651.
- ↑ Rappuoli R, Nicosia A, Perugini M, Franzini C, Casagli MC, Borri MG, Antoni G, Almoni M, Neri P, Ratti G (1986). "Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication". Proc. Natl. Acad. Sci. U.S.A. 83 (13): 4631–4635. doi:10.1073/pnas.83.13.4631. PMC 323795
 . PMID 2873570.
. PMID 2873570. - ↑ Cherry JD (March 2007). "Historical Perspective on Pertussis and Use of Vaccines to Prevent It". Microbe Magazine.
- ↑ Parton R (June 1999). "Review of the biology of Bordetella pertussis". Biologicals. 27 (2): 71–6. doi:10.1006/biol.1999.0182. PMID 10600186.
- 1 2 Gibert M, Perelle S, Boquet P, Popoff MR (1993). "Characterization of Clostridium perfringens iota-toxin genes and expression in Escherichia coli". Infect. Immun. 61 (12): 5147–5156. PMC 281295
 . PMID 8225592.
. PMID 8225592. - 1 2 Kaslow HR, Burns DL (June 1992). "Pertussis toxin and target eukaryotic cells: binding, entry, and activation". FASEB J. 6 (9): 2684–90. PMID 1612292.
- 1 2 Locht C, Antoine R (1995). "A proposed mechanism of ADP-ribosylation catalyzed by the pertussis toxin S1 subunit". Biochimie. 77 (5): 333–40. doi:10.1016/0300-9084(96)88143-0. PMID 8527486.
- ↑ Weiss A, Johnson F, Burns D (1993). "Molecular characterization of an operon required for pertussis toxin secretion". Proc Natl Acad Sci U S A. 90 (7): 2970–4. doi:10.1073/pnas.90.7.2970. PMC 46218
 . PMID 8464913.
. PMID 8464913. - ↑ Plaut RD, Carbonetti NH (May 2008). "Retrograde transport of pertussis toxin in the mammalian cell". Cell. Microbiol. 10 (5): 1130–9. doi:10.1111/j.1462-5822.2007.01115.x. PMID 18201245.
- ↑ Finger H, von Koenig CH (1996). "Bordetella". In Barron S, et al. Barron's Medical Microbiology (4th ed.). Univ of Texas Medical Branch. ISBN 0-9631172-1-1.
- ↑ Burns D (1988). "Subunit structure and enzymic activity of pertussis toxin". Microbiol Sci. 5 (9): 285–7. PMID 2908558.
- ↑ Carbonetti NH (2010). "Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools". Future Microbiol. 5 (3): 455–69. doi:10.2217/fmb.09.133. PMC 2851156
 . PMID 20210554.
. PMID 20210554. - ↑ Bestebroer, J., de Haas, C.J.C. & van Strijp, J.A.G. (2010). "How microorganisms avoid phagocyte attraction". FEMS Microbiology Reviews. 34 (3): 395–414. doi:10.1111/j.1574-6976.2009.00202.x. PMID 20059549.
- 1 2 Andreasen, C. & Carbonetti, N.H. (2008). "Pertussis Toxin Inhibits Early Chemokine Production To Delay Neutrophil Recruitment in Response to Bordetella pertussis Respiratory Tract Infection in Mice". Infection and Immunity. 76 (11): 5139–5148. doi:10.1128/IAI.00895-08. PMC 2573337
 . PMID 18765723.
. PMID 18765723. - ↑ Cherry, J.D.; Baraff, LJ; Hewlett, E (1989). "The past, present, and future of pertussis. The role of adults in epidemiology and future control". Western Journal of Medicine. 150 (3): 319–328. PMC 1026455
 . PMID 2660414.
. PMID 2660414. - ↑ Kügler S, Böcker K, Heusipp G, Greune L, Kim KS, Schmidt MA (March 2007). "Pertussis toxin transiently affects barrier integrity, organelle organization and transmigration of monocytes in a human brain microvascular endothelial cell barrier model". Cell. Microbiol. 9 (3): 619–32. doi:10.1111/j.1462-5822.2006.00813.x. PMID 17002784.
- ↑ Weber MS, Benkhoucha M, Lehmann-Horn K, et al. (2010). Unutmaz D, ed. "Repetitive Pertussis Toxin Promotes Development of Regulatory T Cells and Prevents Central Nervous System Autoimmune Disease". PLoS ONE. 5 (12): e16009. doi:10.1371/journal.pone.0016009. PMC 3012729
 . PMID 21209857.
. PMID 21209857. - 1 2 Pande, A.H., Moe, D., Jamnadas, M., Tatulian, S.A. & Teter, K. (2006). "The Pertussis Toxin S1 Subunit Is a Thermally Unstable Protein Susceptible to Degradation by the 20S Proteasome". Biochemistry. 45 (46): 13734–40. doi:10.1021/bi061175+. PMC 2518456
 . PMID 17105192.
. PMID 17105192.
- Stein PE, Boodhoo A, Armstrong GD, Cockle SA, Klein MH, Read RJ (January 1994). "The crystal structure of pertussis toxin". Structure. 2 (1): 45–57. doi:10.1016/S0969-2126(00)00007-1. PMID 8075982.