Patent Blue V
 | |
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
| 3536-49-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 69520 |
| ECHA InfoCard | 100.039.671 |
| E number | E131 (colours) |
| |
| |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
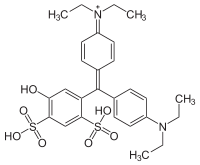
Patent Blue V, also called Food Blue 5, Sulphan Blue, Acid Blue 3, L-Blau 3, C-Blau 20, Patentblau V, Sky Blue, or C.I. 42051 and is a dark bluish synthetic triphenylmethane dye used as a food coloring. As a food additive, it has E number E131. It is a sodium or calcium salt of [4-(α-(4-diethylaminophenyl)-5-hydroxy- 2,4-disulfophenylmethylidene)-2,5-cyclohexadien-1-ylidene] diethylammonium hydroxide inner salt.
It is not widely used, but in Europe it can be found in Scotch eggs, certain jelly sweets, blue Curaçao, certain jello varieties (though not in actual Jell-O brand products), among others. An important advantage is the very deep color it produces even at low concentration, a disadvantage is that it fades fairly quickly when exposed to light.
Patent Blue V is banned as a food dye in Australia and US, because health officials in these countries suspect that it may cause allergic reactions, with symptoms ranging from itching[1] and nettle rash to nausea, hypotension, and in rare cases anaphylactic shock; it is therefore not recommended for children in those countries.
In medicine, Patent Blue V is used in lymphangiography and sentinel node biopsy as a dye to color lymph vessels.[2] It is also used in dental disclosing tablets as a stain to show dental plaque on teeth.
The color of the dye is pH-dependent. In aqueous solution, its color will vary from a deep blue in alkaline or weakly acidic medium to a yellow–orange in stronger acidic conditions. It is useful as a pH indicator for the range 0.8–3.0.[3] The structure is also redox-sensitive, changing from a reduced yellow form to an oxidized red form in solution. The reduction potential of around 0.77 volts is similar to that of other triphenylmethane dyes. It is usable as a reversible redox indicator in some analytical methods.[4]
Because of its pH-dependent color, Patent Blue V was included in chemistry sets from Salter Science in the 1970s and 80s under the name Sky Blue.
References
- ↑ L. Barthelmes, A. Goyal, R.G. Newcombe, F. McNeill, R.E. Mansel, Adverse reactions to patent blue V dye – The NEW START and ALMANAC experience, European Journal of Surgical Oncology (EJSO), Volume 36, Issue 4, April 2010, Pages 399-403, ISSN 0748-7983
- ↑ Erratum - 44 (4): 649 - The Journal of Nuclear Medicine
- ↑ Sabnis, R. W. (2008). "Patent Blue V". Handbook of Acid–Base Indicators. CRC Press. pp. 295–297.
- ↑ Yoe, John H.; Boyd, George R. (1939). "Patent blue V as a pH and redox indicator". Ind. Eng. Chem. Anal. Ed. 11 (9): 492–493. doi:10.1021/ac50137a008.