Paramethadione
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| ATC code | N03AC01 (WHO) |
| Pharmacokinetic data | |
| Protein binding | Not significant |
| Identifiers | |
| |
| CAS Number |
115-67-3 |
| PubChem (CID) | 8280 |
| IUPHAR/BPS | 7261 |
| DrugBank |
DB00617 |
| ChemSpider |
7979 |
| UNII |
Z615FRW64N |
| KEGG |
D00495 |
| ChEMBL |
CHEMBL1100 |
| Chemical and physical data | |
| Formula | C7H11NO3 |
| Molar mass | 157.167 g/mol |
| 3D model (Jmol) | Interactive image |
| Chirality | Racemic mixture |
| |
| |
| (verify) | |
Paramethadione (brand name Paradione) is an anticonvulsant in the oxazolidinedione class developed by by the Illinois based pharmaceutical company Abbott Laboratories (known as AbbVie since January 1, 2013 [1]), and approved by the Food and Drug Administration in 1949 for the treatment of absence seizures, also called partial seizures.[2][3]
In 1960, the yearly cost for 900 mg/day paramethadione was approximately $66,[4] which would translate to $462 yearly in 2007 (with CPI inflation) if paramethadione was still sold.[5]
Mechanism of Action
Paramethadione acts to reduce T-type calcium currents in thalamic neurons which has been proposed to to underlie the the 3-Hz spike-and-wave discharge seen on electroencephalogram (EEG) during absence seizures. [6] [7]
Adverse Effects
Paramethadione is associated with various adverse effects including sedation, increased visual sensitivity to light, GI distress, edema, nephropathy, neutropenia, myasthenia gravis-like syndrome, fatal aplastic anemia, and sever birth defects known as fetal trimethadione syndrome (or paramethadione syndrome).[8][9]
History, society, and culture
FDA approval
Paramethadione (brand name Paradione) was originally approved by the U.S. Food and Drug Administration (FDA) in 1949, as a second-line treatment for petit mal and absence seizures.[10] Paramethadione was ultimately discontinued in 1994 due to safety and efficacy concerns,[11][12] such as being associated with with fetal trimethadione syndrome, which is also known as paramethadione syndrome.[13]
Patents
Paramethadione was first patented in 1949 (U.S. Patent 2,575,693) by Abbott Laboratories.[14] Abbott Labbortories continued to hold the patent to paramethadione until the most recent 1982 approval[15] was withdrawn in in 2004 due to the drug no longer being in use.[16]
Clinical Trials
In the 1940's trimethadione (brand name Tridione) was the only available treatment for absence seizures. However, while effective, this drug presented with significant adverse effects, which led to the search for an equally effective analog. While limited information is available from the time, a pre-market clinical study found that paramethadione, an analog of trimethadione, was not quite as effective at alleviating seizures as trimethadione, however, it did have a significantly lower side effect profile in 85 patients over the course of 2 years. [17] Notably, 80% of patients still showed a good response to paramethadione. [18]
Chemistry
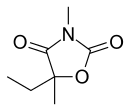
Paramethadione, 5-ethyl-3,5-dimethyloxazolidine-2,4-dione, differs from trimethadione only in the substitution of one methyl group with an ethyl group. It is synthesized in a completely analogous manner, except that it comes from 2-hydroxy-2-methylbutyric acid instead of 2-hydroxyisobutyric acid.
- M.A. Spielman, U.S. Patent 2,575,693 (1951).
References
- ↑ More than splitting pills: Health care giant Abbott Laboratories ready to spin off AbbVie - Retrieved November 7, 2016
- ↑ Oxazolidinedione Anticonvulsants - Retrieved January 2007.
- ↑ Sittig, Marshall (2015). Pharmaceutical manufacturing encyclopedia. New York: William Andrew Pub.
- ↑ Lennox, W.G. (1960). Epilepsy and related disorders. Boston: Little Brown.
- ↑ Shorvon, S.D. (2009). "Drug treatment of epilepsy in the century of the ILAE: The second 50 years, 1959–2009". Epilepsia. 50 (s3): 93–130. doi:10.1111/j.1528-1167.2009.02042.x.
- ↑ Drug Bank- Paramethadione Retrieved November 21, 2016
- ↑ Von Krosigk, M.; Bal, T.; McCormick, D. A. (2009). "Cellular mechanisms of a synchronized oscillation in the thalamus". Science-New York then Washington-. 261: 361–361. JSTOR 2881575.
- ↑ Miller, R.R.; Greenblatt, D.J. (1979). "Handbook of Drug Therapy". New York: Elsevier North Holland.
- ↑ Multiple Congenital Anomaly/Mental Retardation (MCA/MR) Syndromes - Retrieved January 2007.
- ↑ Miller, R.R.; Greenblatt, D.J. (1979). Handbook of Drug Therapy. New York: Elsevier North Holland. p. 597.
- ↑ Drug information for PARADIONE
- ↑ Shorvon, S.D. (2009). "Drug treatment of epilepsy in the century of the ILAE: The second 50 years, 1959–2009". Epilepsia. 50 (s3): 93–130. doi:10.1111/j.1528-1167.2009.02042.x.
- ↑ Multiple Congenital Anomaly/Mental Retardation (MCA/MR) Syndromes - Retrieved January 2007.
- ↑ M.A. Spielman, U.S. Patent 2,575,693 (1951)
- ↑ FDA Orange Book 6800 - Retrieved November 10, 2016
- ↑ Schering Corp.; et al. (2004). Withdrawal of Approval of 92 New Drug Applications and 49 Abbreviated New Drug Applications (Report). DEPARTMENT OF HEALTH AND HUMAN SERVICES: Food and Drug Administration. p. 25125. Docket No. 2004N-0159.
- ↑ Davis, J.P.; Davis, W.G (1949). "A comparison of paradione and tridione in the treatment of epilepsy". The Journal of Pediatrics. 34 (3): 273–278. doi:10.1016/S0022-3476(49)80080-1.
- ↑ Ebaugh, F.G.; Drake, F.R. (1955). "The current drug therapy of epilepsy: a review.". The American journal of the medical sciences. 230 (1): 98.
External links
- Hoffman, D; Chun A (1975). "Paramethadione and metabolite serum levels in humans after a single oral paramethadione dose". J Pharm Sci. 64 (10): 1702–1703. doi:10.1002/jps.2600641027. PMID 1185541.