Pamidronic acid
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| MedlinePlus | a601163 |
| Pregnancy category | |
| Routes of administration | Intravenous |
| ATC code | M05BA03 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | n/a |
| Protein binding | 54% |
| Metabolism | Nil |
| Biological half-life | 28 ± 7 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
40391-99-9 |
| PubChem (CID) | 4674 |
| IUPHAR/BPS | 7259 |
| DrugBank |
DB00282 |
| ChemSpider |
4512 |
| UNII |
OYY3447OMC |
| KEGG |
D07281 |
| ChEMBL |
CHEMBL834 |
| ECHA InfoCard | 100.049.897 |
| Chemical and physical data | |
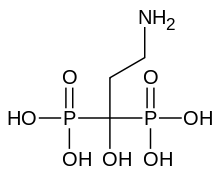
| Formula | C3H11NO7P2 |
| Molar mass | 235.07 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Pamidronic acid (INN) or pamidronate disodium (USAN), pamidronate disodium pentahydrate is a nitrogen-containing bisphosphonate, used to prevent osteoporosis. It is marketed by Novartis under the brand name Aredia. In India, it is marketed by Curacell Biotech under the brand name Pamimed.
Uses
It is used to prevent bone loss, and treat osteoporosis. It is also used to strengthen bone in Paget's disease, to prevent bone loss due to steroid use, and in certain cancers with high propensity to bone, such as multiple 2 myeloma. In multiple 22,2 22 myeloma, it is usually administered as an intravenous infusion, lasting about 3 hours. The therapy is repeated monthly, and lasts for the life of the patient. Due to its ability to sequester calcium in bone, it is also used to treat high calcium levels. It is also used as an experimental treatment of the bone disorder known as Osteogenesis Imperfecta. It has been studied in the treatment of Complex Regional Pain Syndrome.[1]
Administration
Intravenous, usually 90 mg monthly. 30 mg, 60 mg, 90 mg and for hospitals, 120 mg vials are available, mixed with mannitol.
Side effects
Common side effects include bone pain, low calcium levels, nausea, and dizziness. Osteonecrosis of the jaw is a rare complication which has been associated with the use of bisphosphonates, including pamidronate.[2]
Pamidronate activates human γδ T cells in vitro and in vivo, which may lead to flu-like symptoms upon administration.
References
- ↑ I. Kubalek; O. Fain; J. Paries; A. Kettaneh; M. Thomas (2001). "Treatment of reflex sympathetic dystrophy with pamidronate: 29 cases.". Rheumatology. 40 (12): 1394–1397. doi:10.1093/rheumatology/40.12.1394. PMID 11752511.
- ↑ Zarychanski R, Elphee E, Walton P, Johnston J (2006). "Osteonecrosis of the jaw associated with pamidronate therapy.". Am J Hematol. 81 (1): 73–5. doi:10.1002/ajh.20481. PMID 16369966.