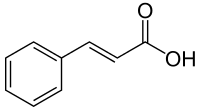
p-Coumaric acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(E)-3-(4-hydroxyphenyl)-2-propenoic acid | |
| Other names
para-coumaric acid,
4-hydroxycinnamic acid, β-(4-hydroxyphenyl)acrylic acid | |
| Identifiers | |
| 501-98-4 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChEBI | CHEBI:32374 |
| ChEMBL | ChEMBL66879 |
| ChemSpider | 553148 |
| DrugBank | DB04066 |
| ECHA InfoCard | 100.116.210 |
| EC Number | 231-000-0 |
| 5787 | |
| PubChem | 637542 |
| |
| |
| Properties | |
| C9H8O3 | |
| Molar mass | 164.16 g·mol−1 |
| Melting point | 210 to 213 °C (410 to 415 °F; 483 to 486 K) |
| Hazards | |
| R-phrases | R36/37/38 |
| S-phrases | S24/25 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
p-Coumaric acid is a hydroxycinnamic acid, an organic compound that is a hydroxy derivative of cinnamic acid. There are three isomers of coumaric acid—o-coumaric acid, m-coumaric acid, and p-coumaric acid—that differ by the position of the hydroxy substitution of the phenyl group. p-Coumaric acid is the most abundant isomer of the three in nature. p-Coumaric acid exists in two forms trans-p-coumaric acid and cis-p-coumaric acid.
It is a crystalline solid that is slightly soluble in water, but well soluble in ethanol and diethyl ether.
Together with sinapyl alcohol and coniferyl alcohols, p-coumaric acid is a major component of lignin.
Natural occurrences
p-Coumaric acid can be found in Gnetum cleistostachyum.[1]
In food
p-Coumaric acid can be found in a wide variety of edible plants such as peanuts, navy beans, tomatoes, carrots, basil and garlic. It is found in wine and vinegar.[2] It is also found in barley grain.[3]
p-Coumaric acid from pollen is a constituent of honey.[4]
Derivatives
p-Coumaric acid glucoside can also be found in commercial breads containing flaxseed.[5]
Diesters of p-coumaric acid can be found in carnauba wax.
Metabolism
Biosynthesis
It is biosynthesized from cinnamic acid by the action of the P450-dependent enzyme 4-cinnamic acid hydroxylase (C4H).
It is also produced from L-tyrosine by the action of tyrosine ammonia lyase (TAL).
-

 + Ammonia + H+
+ Ammonia + H+
Biochemistry
p-Coumaric acid is the precursor of 4-ethylphenol produced by the yeast Brettanomyces in wine. The yeast converts this to 4-vinylphenol via the enzyme cinnamate decarboxylase.[6] 4-Vinylphenol is further reduced to 4-ethylphenol by the enzyme vinyl phenol reductase. Coumaric acid is sometimes added to microbiological media, enabling the positive identification of Brettanomyces by smell.
 The conversion of p-coumaric acid to 4-ethyphenol by Brettanomyces
The conversion of p-coumaric acid to 4-ethyphenol by Brettanomyces
cis-p-coumarate glucosyltransferase is an enzyme that uses UDP-glucose and cis-p-coumarate to produce 4'-O-beta-D-glucosyl-cis-p-coumarate and UDP. This enzyme belongs to the family of glycosyltransferases, specifically the hexosyltransferases.[7]
Phloretic acid is found in the rumen of sheep fed with dried grass and is produced by hydrogenation of the 2-propenoic side chain of p-coumaric acid.[8]
Medicinal uses
p-Coumaric acid has antioxidant properties and is believed to reduce the risk of stomach cancer[9] by reducing the formation of carcinogenic nitrosamines.[10] p-Coumaric acid in honey also demonstrate in vitro anti-inflammatory activity[11]
Biological Effects
p-Coumaric acid is normally present in honey, but not in the substitute nutrients based on high-fructose corn syrup that honey bee keepers feed to their colonies. This absence has been suggested as a possible contributor to Colony Collapse Disorder of honey bees because p-coumaric acid has been found to help honey bees detoxify certain pesticides.[4]
See also
- Coumarin
- Coumaroyl-Coenzyme A
- Ferulic acid
- Cinnamic acid
- Phenolic content in wine
- p-Coumaroylated anthocyanins
References
- ↑ Yao, Chun-Suo; Lin, Mao; Liu, Xin; Wang, Ying-Hong (2005). "Stilbene derivatives from Gnetum cleistostachyum". Journal of Asian Natural Products Research. 7 (2): 131–7. doi:10.1080/10286020310001625102. PMID 15621615.
- ↑ Gálvez, Miguel Carrero; Barroso, Carmelo García; Pérez-Bustamante, Juan Antonio (1994). "Analysis of polyphenolic compounds of different vinegar samples". Zeitschrift für Lebensmittel-Untersuchung und -Forschung. 199: 29–31. doi:10.1007/BF01192948.
- ↑ Quinde-Axtell, Zory; Baik, Byung-Kee (2006). "Phenolic Compounds of Barley Grain and Their Implication in Food Product Discoloration". Journal of Agricultural and Food Chemistry. 54 (26): 9978–84. doi:10.1021/jf060974w. PMID 17177530.
- 1 2 Mao W, Schuler MA, Berenbaum MR (2013). "Honey constituents up-regulate detoxification and immunity genes in the western honey bee Apis mellifera". Proceedings of the National Academy of Sciences of the United States of America. 110 (April 29, 2013): 8842–6. doi:10.1073/pnas.1303884110. PMC 3670375
 . PMID 23630255.
. PMID 23630255. - ↑ Strandås, C.; Kamal-Eldin, A.; Andersson, R.; Åman, P. (2008). "Phenolic glucosides in bread containing flaxseed". Food Chemistry. 110 (4): 997–999. doi:10.1016/j.foodchem.2008.02.088.
- ↑ Brettanomyces Monitoring by Analysis of 4-ethylphenol and 4-ethylguaiacol at etslabs.com
- ↑ Rasmussen, Susanne; Rudolph, Hansjörg (1997). "Isolation, purification and characterization of UDP-glucose: Cis-p-coumaric acid-β-d-glucosyltransferase from sphagnum fallax". Phytochemistry. 46 (3): 449–453. doi:10.1016/S0031-9422(97)00337-3.
- ↑ Chesson, A; Stewart, CS; Wallace, RJ (1982). "Influence of plant phenolic acids on growth and cellulolytic activity of rumen bacteria". Applied and Environmental Microbiology. 44 (3): 597–603. PMC 242064
 . PMID 16346090.
. PMID 16346090. - ↑ Ferguson LR, Shuo-tun Z, Harris PJ (2005). "Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29". Molecular Nutrition & Food Research. 49 (6): 585–693. doi:10.1002/mnfr.200500014. PMID 15841493.
- ↑ Kikugawa K, Hakamada T, Hasunuma M, Kurechi T (1983). "Reaction of p-hydroxycinnamic acid derivatives with nitrite and its relevance to nitrosamine formation". Journal of Agricultural and Food Chemistry. 1 (4): 780–785. doi:10.1021/jf00118a025.
- ↑ Kassim, Mustafa; et al. "Ellagic acid, phenolic acids, and flavonoids in Malaysian honey extracts demonstrate in vitro anti-inflammatory activity".