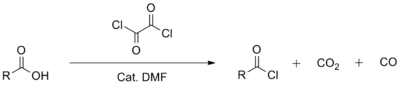Oxalyl chloride
 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Oxalyl dichloride[1] | |||
| Systematic IUPAC name
Ethanedioyl dichloride[1] | |||
| Other names
Oxalic acid chloride Oxalic acid dichloride Oxalic dichloride Oxaloyl chloride | |||
| Identifiers | |||
| 79-37-8 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 59021 | ||
| ECHA InfoCard | 100.001.092 | ||
| EC Number | 201-200-2 | ||
| PubChem | 65578 | ||
| RTECS number | KI2950000 | ||
| |||
| |||
| Properties | |||
| C2O2Cl2 | |||
| Molar mass | 126.93 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 1.4785 g/mL | ||
| Melting point | −16 °C (3 °F; 257 K) | ||
| Boiling point | 63 to 64 °C (145 to 147 °F; 336 to 337 K) at 1.017 bar | ||
| Reacts | |||
| Refractive index (nD) |
1.429 | ||
| Hazards | |||
| Main hazards | Toxic, corrosive, lachrymator [2] | ||
| Safety data sheet | External MSDS | ||
| GHS pictograms |   [2] [2] | ||
| GHS signal word | Danger[2] | ||
| H314, H331[2] | |||
| P261, P280, P305+351+338, P310[2] | |||
| EU classification (DSD) |
| ||
| R-phrases | R14 R23 R29 R34 [2] | ||
| S-phrases | (S1/2) S26 S30 S36/37/39 S38 S45 S61 [2] | ||
| NFPA 704 | |||
| Related compounds | |||
| Related acyl chlorides |
Malonyl chloride Succinyl chloride phosgene | ||
| Related compounds |
Oxalic acid Diethyl oxalate Oxamide Oxalyl hydrazide Cuprizon 1 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Oxalyl chloride is a chemical compound with the formula (COCl)2. This colourless, sharp-smelling liquid, the diacid chloride of oxalic acid, is a useful reagent in organic synthesis.[3] It can be prepared by treating oxalic acid with phosphorus pentachloride.[4]
Reactions
Oxalyl chloride reacts with water giving off gaseous products only: hydrogen chloride (HCl), carbon dioxide (CO
2), and carbon monoxide (CO).
- (COCl)2 + H
2O → 2 HCl + CO
2 + CO
In this, it is quite different from other acyl chlorides which hydrolyze with formation of hydrogen chloride and the original carboxylic acid.
Applications in organic synthesis
Oxidation of alcohols
The solution comprising DMSO and oxalyl chloride, followed by quenching with triethylamine converts alcohols to the corresponding aldehydes and ketones via the process known as the Swern oxidation.[5][6][7]
Synthesis of acyl chlorides
Oxalyl chloride is mainly used together with a N,N-dimethylformamide catalyst in organic synthesis for the preparation of acyl chlorides from the corresponding carboxylic acids. Like thionyl chloride, the reagent degrades in volatile side products in this application, which simplifies workup. One of the minor by-products from this reaction is a potent carcinogen.[8] Relative to thionyl chloride, oxalyl chloride tends to be a milder, more selective reagent. It is also more expensive than thionyl chloride so it tends to be used on a smaller scale.
This reaction involves conversion of DMF to the imidoyl chloride derivative (Me2N=CHCl+), akin to the first stage in the Vilsmeier–Haack reaction. The imidoyl chloride is the active chlorinating agent.
Formylation of arenes
Oxalyl chloride reacts with aromatic compounds in the presence of aluminium chloride to give the corresponding acyl chloride in a process known as a Friedel-Crafts acylation.[9][10] The resulting acyl chloride can be hydrolysed in water to form the corresponding carboxylic acid.
Preparation of oxalate diesters
Like other acyl chlorides, oxalyl chloride reacts with alcohols to give esters:
- 2 RCH2OH + (COCl)2 → RCH2OC(O)C(O)OCH2R + 2 HCl
Typically, such reactions are conducted in the presence of a base such as pyridine. The diester derived from phenol, phenyl oxalate ester, is Cyalume, the active ingredient in glow sticks.
Other
Oxalyl chloride was reportedly used in the first synthesis of dioxane tetraketone (C4O6), an oxide of carbon.[11]
Precautions
In March 2000, a Malaysia Airlines Airbus A330 was damaged beyond repair after a falsely declared cargo of oxalyl chloride leaked into the cargo bay.[12]
See also
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 797. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 6 7 8 Oxalyl chloride MSDS
- ↑ Salmon, R. (2001). "Oxalyl Chloride". Encyclopedia of Reagents for Organic Synthesis. New York: John Wiley & Sons. doi:10.1002/047084289X.ro015.
- ↑ DE patent 2840435, Vogel, A.; Steffan, G.; Mannes, K.; Trescher, V., "Process for the preparation of oxalyl chloride", issued 1980-03-27, assigned to Bayer
- ↑ Dondoni, A.; Perrone, D. (2004). "Synthesis of 1,1-Dimethyl Ethyl-(S)-4-formyl-2,2-dimethyl-3-oxazolidinecarboxylate by Oxidation of the Alcohol". Org. Synth.; Coll. Vol., 10, p. 320
- ↑ Bishop, R. (1998). "9-Thiabicyclo[3.3.1]nonane-2,6-dione". Org. Synth.; Coll. Vol., 9, p. 692
- ↑ Leopold, E. J. (1990). "Selective hydroboration of a 1,3,7-triene: Homogeraniol". Org. Synth.; Coll. Vol., 7, p. 258
- ↑ Clayden, Jonathan (2005). Organic chemistry (Reprinted (with corrections). ed.). Oxford [u.a.]: Oxford Univ. Press. p. 296. ISBN 978-0-19-850346-0.
- ↑ Neubert, M. E.; Fishel, D. L. (1983). "Preparation of 4-Alkyl- and 4-Halobenzoyl Chlorides: 4-Pentylbenzoyl Chloride". Org. Synth. 61: 8.; Coll. Vol., 7, p. 420
- ↑ Sokol, P. E. (1964). "Mesitoic Acid". Org. Synth. 44: 69.; Coll. Vol., 5, p. 706
- ↑ Strazzolini, P.; Gambi, A.; Giumanini, A. G.; Vancik, H. (1998). "The reaction between ethanedioyl (oxalyl) dihalides and Ag2C2O4: a route to Staudinger's elusive ethanedioic (oxalic) acid anhydride". Journal of the Chemical Society, Perkin Transactions 1. 1998 (16): 2553–2558. doi:10.1039/a803430c.
- ↑ "Firm told to pay $65 mln for ruining plane". Reuters. 2007-12-06. Retrieved 2007-12-06.