Orthosilicate (ion)
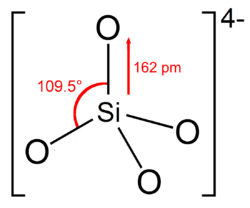
The orthosilicate ion, or silicon tetroxide anion, is SiO4−
4. The name of the ion is frequently simplified to silicate, as it forms the basis of most mineral-forming silicate oxoanions. An orthosilicate compound is a compound that contains this group.
In its ionic form, orthosilicate is difficult to study. It is an extremely strong base and does not persist in solution, tending to be consumed by water to form extremely weak silicic acid (H4SiO4; pKa2 = 13.2 at 25 °C), a very difficult-to-control reaction that typically leaves only minuscule amounts of the ion and large amounts of hydrated silica condensate.[1] Its salts, however, are extremely abundant in the form of nesosilicate minerals.[2] Olivine, which may be referred to as magnesium or iron(II) (ortho-)silicate, is the most abundant mineral in the earth's mantle.
The ion has a classic tetrahedral shape, with one silicon surrounded by four oxygen centres.[3] The Si–O bond is 162 pm long.[4]
Organic chemistry
Although very important in inorganic chemistry and geochemistry, the orthosilicate ion is rarely seen in organic chemistry. Two silicate compounds, however, are used in organic synthesis: tetraethyl orthosilicate or TEOS is used to link polymers, and is especially important in the manufacture of aerogels. Tetramethyl orthosilicate or TMOS is used as an alternative to TEOS, and also has a number of other uses as a reagent. TEOS is preferred over TMOS as TMOS decomposes to produce high concentrations of toxic methanol. Inhaling TMOS can result in toxic build-up of silica in the lungs.
References
- ↑ Jurkić, Lela Munjas; Cepanec, Ivica; Pavelić, Sandra Kraljević; Pavelić, Krešimir (2013). "Biological and therapeutic effects of ortho-silicic acid and some ortho-silicic acid-releasing compounds: New perspectives for therapy". Nutrition & Metabolism. 10 (1): 2. doi:10.1186/1743-7075-10-2. ISSN 1743-7075.
- ↑ Western Oregon University
- ↑ Balaram Sahoo; Nayak Nimai Charan; Samantaray Asutosh; Pujapanda Prafulla Kumar. Inorganic Chemistry. PHI Learning Pvt. Ltd. p. 306. ISBN 978-81-203-4308-5.
- ↑ Horacio E. Bergna; William O. Roberts (19 December 2005). Colloidal Silica: Fundamentals and Applications. CRC Press. p. 10. ISBN 978-1-4200-2870-6.