Organic electronics
Organic electronics is a field of materials science concerning the design, synthesis, characterization, and application of organic small molecules or polymers that show desirable electronic properties such as conductivity. Unlike conventional inorganic conductors and semiconductors, organic electronic materials are constructed from organic (carbon-based) small molecules or polymers using synthetic strategies developed in the context of organic and polymer chemistry. One of the promised benefits of organic electronics is their potential low cost compared to traditional inorganic electronics.[1][2] Attractive properties of polymeric conductors include their electrical conductivity that can be varied by the concentrations of dopants. Relative to metals, they have mechanical flexibility. Some have high thermal stability.
History
One class of materials of interest in organic electronics are electrical conductive, i.e. substances that can transmit electrical charges with low resistivity. Traditionally, conductive materials are inorganic. Classical (and still technologically dominant) conductive materials are metals such as copper and aluminum as well as many alloys.[3]
The earliest reported organic conductive material, polyaniline, was described by Henry Letheby in 1862. Work on other polymeric organic materials began in earnest in the 1960s, A high conductivity of 1 S/cm (S = Siemens) was reported in 1963 for a derivative of tetraiodopyrrole.[4] In 1977, it was discovered that polyacetylene can be oxidized with halogens to produce conducting materials from either insulating or semiconducting materials. The 2000 Nobel Prize in Chemistry was awarded to Alan J. Heeger, Alan G. MacDiarmid, and Hideki Shirakawa jointly for their work on conductive polymers.[5] These and many other workers identified large families of electrically conducting polymers including polythiophene, polyphenylene sulfide, and others.
In the 1950s, a second class of electric conductors were discovered based on charge-transfer salts. An early examples were derivatives of polycyclic aromatic compounds. For example, pyrene was shown to form semiconducting charge-transfer complex salts with halogens. In 1972, researchers found metallic conductivity(conductivity comparable to a metal) in the charge-transfer complex TTF-TCNQ.
Conductive plastics have undergone development for applications in industry. In 1987, the first organic diode was produced at Eastman Kodak by Ching W. Tang and Steven Van Slyke.[6]
The initial characterization of the basic properties of polymer light emitting diodes, demonstrating that the light emission phenomenon was injection electroluminescence and that the frequency response was sufficiently fast to permit video display applications, was reported by Bradley, Burroughes, Friend, et al. in a 1990 Nature paper. Moving from molecular to macromolecular materials solved the problems previously encountered with the long-term stability of the organic films and enabled high-quality films to be easily made.[7] Subsequent research developed multilayer polymers and the new field of plastic electronics and organic light-emitting diodes (OLED) research and device production grew rapidly.[8]
Conductive organic materials
Organic conductive materials can be grouped into two main classes: conductive polymers and conductive molecular solids and salts.
Molecular solids and salts
Semiconducting small molecules include polycyclic aromatic compounds such as pentacene and rubrene.
Conductive polymers
Conductive polymers are often typically intrinsically conductive or at least semiconductors. They sometimes show mechanical properties comparable to those of conventional organic polymers. Both organic synthesis and advanced dispersion techniques can be used to tune the electrical properties of conductive polymers, unlike typical inorganic conductors. The most well-studied class of conductive polymers include polyacetylene, polypyrrole, polyaniline, and their copolymers. Poly(p-phenylene vinylene) and its derivatives are used for electroluminescent semiconducting polymers. Poly(3-alkythiophenes) are also a typical material for use in solar cells and transistors.
Organic light-emitting diode
An OLED (organic light-emitting diode) consists of a thin film of organic material that emits light under stimulation by an electric current. A typical OLED consists of an anode, a cathode, OLED organic material and a conductive layer.


Discovery of OLED
André Bernanose[9][10] was the first person to observe electroluminescence in organic materials, and Ching W. Tang,[11] reported fabrication of an OLED device in 1987. The OLED device incorporated a double-layer structure motif consisting of separate hole transporting and electron-transporting layers, with light emission taking place in between the two layers. Their discovery opened a new era of current OLED research and device design.
Classification and current research
OLED organic materials can be divided into two major families: small-molecule-based and polymer-based. Small molecule OLEDs (SM-OLEDs) include organometallic chelates(Alq3),[11] fluorescent and phosphorescent dyes, and conjugated dendrimers. Fluorescent dyes can be selected according to the desired range of emission wavelengths; compounds like perylene and rubrene are often used. Very recently, Dr. Kim J. et al.[12] at University of Michigan reported a pure organic light emitting crystal, Br6A, by modifying its halogen bonding, they succeeded in tuning the phosphorescence to different wavelengths including green, blue and red. By modifying the structure of Br6A, scientists are attempting to achieve a next generation organic light emitting diode. Devices based on small molecules are usually fabricated by thermal evaporation under vacuum. While this method enables the formation of well-controlled homogeneous film; is hampered by high cost and limited scalability.[13] [14]
Polymer light-emitting diodes (PLEDs), similar to SM-OLED, emit light under an applied electric current. Polymer-based OLEDs are generally more efficient than SM-OLEDs requiring a comparatively lower amount of energy to produce the same luminescence. Common polymers used in PLEDs include derivatives of poly(p-phenylene vinylene)[15] and polyfluorene. The emitted color can be tuned by substitution of different side chains onto the polymer backbone or modifying the stability of the polymer. In contrast to SM-OLEDs, polymer-based OLEDs cannot be fabricated through vacuum evaporation, and must instead be processed using solution-based techniques. Compared to thermal evaporation, solution based methods are more suited to creating films with large dimensions. Zhenan Bao.[16] et al. at Stanford University reported a novel way to construct large-area organic semiconductor thin films using aligned single crystalline domains.
Organic field-effect transistor
An Organic field-effect transistor is a field-effect transistor utilizing organic molecules or polymers as the active semiconducting layer. A field-effect transistor (FET) is any semiconductor material that utilizes electric field to control the shape of a channel of one type of charge carrier, thereby changing its conductivity. Two major classes of FET are n-type and p-type semiconductor, classified according to the charge type carried. In the case of organic FETs (OFETs), p-type OFET compounds are generally more stable than n-type due to the susceptibility of the latter to oxidative damage.
Discovery of the OFET
J.E. Lilienfeld[17] first proposed the field-effect transistor in 1930, but the first OFET was not reported until 1987, when Koezuka et al. constructed one using Polythiophene[18] which shows extremely high conductivity. Other conductive polymers have been shown to act as semiconductors, and newly synthesized and characterized compounds are reported weekly in prominent research journals. Many review articles exist documenting the development of these materials.[19][20][21][22][23]
Classification of OFETs and current research
Like OLEDs, OFETs can be classified into small-molecule and polymer-based system. Charge transport in OFETs can be quantified using a measure called carrier mobility; currently, rubrene-based OFETs show the highest carrier mobility of 20–40 cm2/(V·s). Another popular OFET material is Pentacene. Due to its low solubility in most organic solvents, it's difficult to fabricate thin film transistors (TFTs) from pentacene itself using conventional spin-cast or, dip coating methods, but this obstacle can be overcome by using the derivative TIPS-pentacene. Current research focuses more on thin-film transistor (TFT) model, which eliminates the usage of conductive materials. Very recently, two studies conducted by Dr. Bao Z.[16] et al. and Dr. Kim J.[24] et al. demonstrated control over the formation of designed thin-film transistors. By controlling the formation of crystalline TFT, it is possible to create an aligned (as opposed to randomly ordered) charge transport pathway, resulting in enhanced charge mobility.
Organic electronic devices

Organic solar cells could cut the cost of solar power by making use of inexpensive organic polymers rather than the expensive crystalline silicon used in most solar cells. What's more, the polymers can be processed using low-cost equipment such as ink-jet printers or coating equipment employed to make photographic film, which reduces both capital and operating costs compared with conventional solar-cell manufacturing.[25]
Silicon thin film solar cells on flexible substrates allow a significant cost reduction of large-area photovoltaics for several reasons:[26]
- The so-called 'roll-to-roll'-deposition on flexible sheets is much easier to realize in terms of technological effort than deposition on fragile and heavy glass sheets.
- Transport and installation of lightweight flexible solar cells also saves cost as compared to cells on glass.
Inexpensive polymeric substrates like polyethylene terephthalate (PET) or polycarbonate (PC) have the potential for further cost reduction in photovoltaics. Protomorphous solar cells prove to be a promising concept for efficient and low-cost photovoltaics on cheap and flexible substrates for large-area production as well as small and mobile applications.[26]
One advantage of printed electronics is that different electrical and electronic components can be printed on top of each other, saving space and increasing reliability and sometimes they are all transparent. One ink must not damage another, and low temperature annealing is vital if low-cost flexible materials such as paper and plastic film are to be used. There is much sophisticated engineering and chemistry involved here, with iTi, Pixdro, Asahi Kasei, Merck & Co.|Merck, BASF, HC Starck, Hitachi Chemical and Frontier Carbon Corporation among the leaders.[27] Electronic devices based on organic compounds are now widely used, with many new products under development. Sony reported the first full-color, video-rate, flexible, plastic display made purely of organic materials;[28][29] television screen based on OLED materials; biodegradable electronics based on organic compound and low-cost organic solar cell are also available.
Fabrication methods
There are important differences between the processing of small molecule organic semiconductors and semiconducting polymers. Small molecule semiconductors are quite often insoluble and typically require deposition via vacuum sublimation. While usually thin films of soluble conjugated polymers. Devices based on conductive polymers can be prepared by solution processing methods. Both solution processing and vacuum based methods produce amorphous and polycrystalline films with variable degree of disorder. "Wet" coating techniques require polymers to be dissolved in a volatile solvent, filtered and deposited onto a substrate. Common examples of solvent-based coating techniques include drop casting, spin-coating, doctor-blading, inkjet printing and screen printing. Spin-coating is a widely used technique for small area thin film production. It may result in a high degree of material loss. The doctor-blade technique results in a minimal material loss and was primarily developed for large area thin film production. Vacuum based thermal deposition of small molecules requires evaporation of molecules from a hot source. The molecules are then transported through vacuum onto a substrate. The process of condensing these molecules on the substrate surface results in thin film formation. Wet coating techniques can in some cases be applied to small molecules depending on their solubility.
Organic solar cells
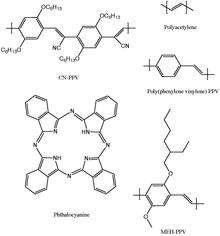
Compared to conventional inorganic solar cell, organic solar cells have the advantage of lower fabrication cost. An organic solar cell is a device that uses organic electronics to convert light into electricity. Organic solar cells utilize organic photovoltaic materials, organic semiconductor diodes that convert light into electricity. Figure to the right shows five commonly used organic photovoltaic materials. Electrons in these organic molecules can be delocalized in a delocalized π orbital with a corresponding π* antibonding orbital. The difference in energy between the π orbital, or highest occupied molecular orbital(HOMO), and π* orbital, or lowest unoccupied molecular orbital(LUMO) is called the band gap of organic photovoltaic materials. Typically, the band gap lies in the range of 1-4eV.[30][31][32]
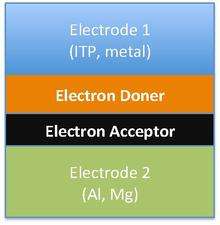
The difference in the band gap of organic photovoltaic materials leads to different chemical structures and forms of organic solar cells. Different forms of solar cells includes single-layer organic photovoltaic cells, bilayer organic photovoltaic cells and heterojunction photovoltaic cells. However, all three of these types of solar cells share the approach of sandwiching the organic electronic layer between two metallic conductors, typically indium tin oxide.[33]

Organic field-effect transistors
An organic field-effect transistor device consists of three major components: the source, the drain and the gate. Generally, a field-effect transistor has two plates, source in contact with drain and the gate respectively, working as conducting channel. The electrons move from source to the drain, and the gate serves to control the electrons’ movement from source to drain. Different types of FETs are designed based on carrier properties. Thin film transistor (TFT), among them, is an easy fabricating one. In a thin film transistor, the source and drain are made by directly depositing a thin layer of semiconductor followed by a thin film of insulator between semiconductor and the metal gate contact. Such a thin film is made by either thermal evaporation, or simply spins coating. In a TFT device, there is no carrier movement between the source and drain. After applying a positive charge, accumulation of electrons on the interface cause bending of the semiconductor and ultimately lowers the conduction band with regards to the Fermi-level of the semiconductor. Finally, a highly conductive channel is formed at the interface.[34]
Features
Conductive polymers are lighter, more flexible, and less expensive than inorganic conductors. This makes them a desirable alternative in many applications. It also creates the possibility of new applications that would be impossible using copper or silicon.
Organic electronics not only includes organic semiconductors, but also organic dielectrics, conductors and light emitters.
New applications include smart windows and electronic paper. Conductive polymers are expected to play an important role in the emerging science of molecular computers.
See also
- Annealing
- Bioplastic
- Carbon nanotube
- Circuit deposition
- Conductive ink
- Disposable electronics
- Flexible display
- Laminar
- Melanin
- Organic field-effect transistor (OFET)
- Organic semiconductor
- Organic light-emitting diode (OLED)
- Photodetector
- Printed electronics
- Radio frequency identification
- Radio tag
- Spin coating
References
- ↑ Hagen Klauk (Ed.) "Organic "Electronics: Materials, Manufacturing and Applications" 2006, Wiley-VCH, Weinheim. Print ISBN 9783527312641.
- ↑ Hagen Klauk (Ed.) "Organic electronics. More materials and applications" 2010, Wiley-VCH, Weinheim. ISBN 9783527640218 electronic bk.
- ↑ "Electrical Conductivity – History". Net Industries and its LicensorsNet Industries and its Licensors.
- ↑ McNeill, R.; Siudak, R.; Wardlaw, J. H.; Weiss, D. E. (1963). "Electronic Conduction in Polymers. I. The Chemical Structure of Polypyrrole". Aust. J. Chem. 16 (6): 1056–1075. doi:10.1071/CH9631056.
- ↑ "The Nobel Prize in Chemistry 2000". Nobelprize.org. Nobel Media.
- ↑ Forrest, S. (2012). "Energy efficiency with organic electronics: Ching W. Tang revisits his days at Kodak". MRS Bulletin. 37 (6): 552. doi:10.1557/mrs.2012.125.
- ↑ "Light-emitting diodes based on conjugated polymers". Nature. 347: 539–541. 1990. doi:10.1038/347539a0.
- ↑ National Research Council (2015). The Flexible Electronics Opportunity. The National Academies Press. pp. 105–6. ISBN 978-0-309-30591-4.
- ↑ Bernanose, A.; Comte, M.; Vouaux, P. (1953). "A new method of light emission by certain organic compounds". J. Chim. Phys. 50: 64.
- ↑ Bernanose, A.; Vouaux, P. (1953). "Organic electroluminescence type of emission". J. Chim. Phys. 50: 261.
- 1 2 Tang, C. W.; Vanslyke, S. A. (1987). "Organic electroluminescent diodes". Applied Physics Letters. 51 (12): 913. Bibcode:1987ApPhL..51..913T. doi:10.1063/1.98799.
- ↑ Kim, Jinsang; Onas Bolton; Kim, Hyong-Jun; Lin, Kevin Y.; Kim, Jinsang (2011). "Activating efficient phosphorescence from purely organic materials by crystal design". Nature Chemistry. 3 (3): 205–210. Bibcode:2011NatCh...3..207B. doi:10.1038/nchem.984. PMID 21336325.
- ↑ Piromreun, Pongpun; Oh, Hwansool; Shen, Yulong; Malliaras, George G.; Scott, J. Campbell; Brock, Phil J. (2000). "Role of CsF on electron injection into a conjugated polymer". Applied Physics Letters. 77 (15): 2403. Bibcode:2000ApPhL..77.2403P. doi:10.1063/1.1317547.
- ↑ Holmes, Russell; Erickson, N.; LüSsem, Björn; Leo, Karl (27 August 2010). "Highly efficient, single-layer organic light-emitting devices based on a graded-composition emissive layer". Applied Physics Letters. 97: 083308. Bibcode:2010ApPhL..97a3308S. doi:10.1063/1.3460285.
- ↑ Burroughes, J. H.; Bradley, D. D. C.; Brown, A. R.; Marks, R. N.; MacKay, K.; Friend, R. H.; Burns, P. L.; Holmes, A. B. (1990). "Light-emitting diodes based on conjugated polymers". Nature. 347 (6293): 539. Bibcode:1990Natur.347..539B. doi:10.1038/347539a0.
- 1 2 Bao, Zhenan; Ying Diao; Giri, Gaurav; Xu, Jie; Kim, Do Hwan; Becerril, Hector A.; Stoltenberg, Randall M.; Lee, Tae Hoon; Xue, Gi; Mannsfeld, Stefan C. B.; Bao, Zhenan (2013). "Solution coating of large-area organic semiconductor thin films with aligned single-crystalline domains". Nature Material. 12 (7): 665–671. Bibcode:2013NatMa..12..665D. doi:10.1038/nmat3650.
- ↑ Lilienfeld, J.E. (1930-01-28). US 1745175 "Method and apparatus for controlling electric currents"
- ↑ Koezuka, H.; Tsumura, A.; Ando, T. (1987). "Field-effect transistor with polythiophene thin film". Synthetic Metals. 18: 699–704. doi:10.1016/0379-6779(87)90964-7.
- ↑ Hasegawa, Tatsuo; Takeya, Jun (2009). "Organic field-effect transistors using single crystals". Sci. Technol. Adv. Mater. (free download). 10 (2): 024314. Bibcode:2009STAdM..10b4314H. doi:10.1088/1468-6996/10/2/024314.
- ↑ Yamashita, Yoshiro (2009). "Organic semiconductors for organic field-effect transistors". Sci. Technol. Adv. Mater. (free download). 10 (2): 024313. Bibcode:2009STAdM..10b4313Y. doi:10.1088/1468-6996/10/2/024313.
- ↑ Dimitrakopoulos, C.D.; Malenfant, P.R.L. (2002). "Organic Thin Film Transistors for Large Area Electronics". Adv. Mater. 14 (2): 99. doi:10.1002/1521-4095(20020116)14:2<99::AID-ADMA99>3.0.CO;2-9.
- ↑ Reese, Colin; Roberts, Mark; Ling, Mang-Mang; Bao, Zhenan (2004). "Organic thin film transistors". Mater. Today. 7 (9): 20. doi:10.1016/S1369-7021(04)00398-0.
- ↑ Klauk, Hagen (2010). "Organic thin-film transistors". Chem. Soc. Rev. 39 (7): 2643. doi:10.1039/B909902F.
- ↑ Kim, Jinsang; Bong-Gi Kim; Chung, Jong Won; Seo, Sungbaek; Koo, Bonwon; Kim, Jinsang (2013). "A molecular design principle of lyotropic liquid-crystalline conjugated polymers with directed alignment capability for plastic electronics". Nature Material. 12 (7): 659–664. Bibcode:2013NatMa..12..659K. doi:10.1038/nmat3595.
- ↑ Bullis, Kevin (October 17, 2008). "Mass Production of Plastic Solar Cells". Technology Review.
- 1 2 Koch, Christian (2002) Niedertemperaturabscheidung von Dünnschicht-Silicium für Solarzellen auf Kunststofffolien, Doctoral Thesis, ipe.uni-stuttgart.de
- ↑ Raghu Das, IDTechEx. "Printed electronics, is it a niche? – 25 September 2008". Electronics Weekly. Retrieved 2010-02-14.
- ↑ プラスチックフィルム上の有機TFT駆動有機ELディスプレイで世界初のフルカラー表示を実現. sony.co.jp (in Japanese)
- ↑ Flexible, full-color OLED display. pinktentacle.com (2007-06-24).
- ↑ Nelson J. (2002). "Organic photovoltaic films". Current Opinion in Solid State and Materials Science. 6: 87–95. Bibcode:2002COSSM...6...87N. doi:10.1016/S1359-0286(02)00006-2.
- ↑ Halls J.J.M. & Friend R.H. (2001). Archer M.D. & Hill R.D., eds. Clean electricity from photovoltaics. London: Imperial College Press. pp. 377–445. ISBN 1860941613.
- ↑ Hoppe, H. and Sarıçiftçi, N. S. (2004). "Organic solar cells: An overview". J. Mater. Res. 19 (7): 1924–1945. Bibcode:2004JMatR..19.1924H. doi:10.1557/JMR.2004.0252.
- ↑ McGehee D.G. & Topinka M.A. (2006). "Solar cells: Pictures from the blended zone". Nature Materials. 5 (9): 675–676. Bibcode:2006NatMa...5..675M. doi:10.1038/nmat1723. PMID 16946723.
- ↑ Weimer, P.K. (1962). "TFT – A New Thin-Film Transistor". Proc. IRE. 50 (6): 1462. doi:10.1109/JRPROC.1962.288190.
External links
- orgworld – Organic Semiconductor World homepage
Further reading
- Grasser, Tibor., Meller, Gregor.Baldo, Marc. (Eds.) (2010) Organic electronics" Springer, Heidelberg. ISBN 978-3-642-04537-0 (Print) 978-3-642-04538-7 (Online)
- Baracus, B. A.; Weiss, D. E. (1963). "Electronic Conduction in Polymers. II. The Electrochemical Reduction of Polypyrrole at Controlled Potential". Aust. J. Chem. 16 (6): 1076–1089. doi:10.1071/CH9631076.
- Bolto, B. A.; McNeill, R.; Weiss, D. E. (1963). "Electronic Conduction in Polymers. III. Electronic Properties of Polypyrrole". Aust. J. Chem. 16 (6): 1090–1103. doi:10.1071/CH9631090.
- Hush, Noel S. (2003). "An Overview of the First Half-Century of Molecular Electronics". Ann. N.Y. Acad. Sci. 1006: 1–20. Bibcode:2003NYASA1006....1H. doi:10.1196/annals.1292.016. PMID 14976006.
- Electronic Processes in Organic Crystals and Polymers, 2 ed. by Martin Pope and Charles E. Swenberg, Oxford University Press (1999), ISBN 0-19-512963-6
- Handbook of Organic Electronics and Photonics (3-Volume Set) by Hari Singh Nalwa, American Scientific Publishers. (2008), ISBN 1-58883-095-0