Octyl acetate
 | |
| Names | |
|---|---|
| IUPAC name
Octyl acetate | |
| Other names
Octyl ethanoate n-Octyl acetate | |
| Identifiers | |
| 112-14-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:87495 |
| ChemSpider | 7872 |
| ECHA InfoCard | 100.003.581 |
| PubChem | 8164 |
| RTECS number | AJ1400000 |
| |
| |
| Properties | |
| C10H20O2 | |
| Molar mass | 172.27 g·mol−1 |
| Appearance | Colorless liquid |
| Odor | Fruity, slightly waxy floral odor |
| Density | 0.863–0.87 g/cm3[1][2] |
| Melting point | −38.5 – −38 °C (−37.3 – −36.4 °F; 234.7–235.2 K)[1][2] |
| Boiling point | 203–211.3 °C (397.4–412.3 °F; 476.1–484.4 K)[1][2] 112.55 °C (234.59 °F; 385.70 K) at 30 mmHg[3][4] |
| 0.021 g/100 g (0 °C) 0.018 g/100 g (29.7 °C) 0.018 g/100 g (40 °C) 0.012 g/100 g (92.1 °C)[5] | |
| Solubility | Soluble in EtOH, ether |
| Vapor pressure | 0.01 kPa (−3 °C) 0.0072–0.0073 (14.75 °C) 0.02–0.1 kPa (27 °C)[3] 1 kPa (66.3 °C) 10 kPa (120 °C)[6] |
| Refractive index (nD) |
1.415–1.422 (20 °C)[3] |
| Thermochemistry | |
| 331–343.74 J/mol·K[4] | |
| Hazards | |
| NFPA 704 | |
| Flash point | 83–86 °C (181–187 °F; 356–359 K)[1][7][8] |
| 268–268.3 °C (514.4–514.9 °F; 541.1–541.5 K)[7][8] | |
| Explosive limits | 0.76–8.14%[7][8] |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
3000 mg/kg (oral, rat)[9] 5000 mg/kg (dermal, rabbit)[9] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
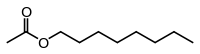
Octyl acetate, or octyl ethanoate, is an organic compound with the formula CH3(CH2)7O2CCH3. It is classified as an ester that is formed from 1-octanol (octyl alcohol) and acetic acid. It is found in oranges, grapefruits, and other citrus products.[10]
Octyl acetate can be synthesized by a condensation of 1-octanol and acetic acid:
- CH3(CH2)7OH + CH3CO2H → CH3(CH2)7O2CCH3 + H2O
Uses
Because of its fruity odor,[11] octyl acetate is used as the basis for artificial flavors and in perfumery. It is also a solvent for nitrocellulose, waxes, oils, and some resins.
References
- 1 2 3 4 Record in the GESTIS Substance Database of the IFA
- 1 2 3 Yaws, Carl L. (2008). Thermophysical Properties of Chemicals and Hydrocarbons. http://www.williamandrew.com. New York: William Andrew, Inc. ISBN 978-0-8155-1596-8. LCCN 2008020146. External link in
|website=(help) - 1 2 3 "Octyl acetate". http://chemdats.blogspot.com. 2014-11-04. Retrieved 2014-11-15. External link in
|website=(help) - 1 2 Acetic acid, octyl ester in Linstrom, P.J.; Mallard, W.G. (eds.) NIST Chemistry WebBook, NIST Standard Reference Database Number 69. National Institute of Standards and Technology, Gaithersburg MD. http://webbook.nist.gov (retrieved 2014-11-22)
- ↑ Stephenson, Richard M. (1992). "Mutual Solubilities: Water-Ketones, Water-Ethers, and Water-Gasoline-Alcohols". Journal of Chemical Engineering Data. 37 (1): 80–95. doi:10.1021/je00005a024.
- ↑ Lide, David R., ed. (2009). CRC Handbook of Chemistry and Physics (90th ed.). Boca Raton, Florida: CRC Press. ISBN 978-1-4200-9084-0.
- 1 2 3 4 "MSDS of Octyl acetate". http://www.fishersci.ca. Fisher Scientific. Retrieved 2014-09-15. External link in
|website=(help) - 1 2 3 Sigma-Aldrich Co., Octyl acetate. Retrieved on 2014-11-15.
- 1 2 Food and Cosmetics Toxicology. 12: 815. 1974. Missing or empty
|title=(help) - ↑ Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- ↑ Brechbill, Glen O. (2007). Classifying Aroma Chemicals. http://www.perfumerbook.com. New Jersey, USA: Fragrance Books, Inc. p. 6. External link in
|website=(help)
This article is issued from Wikipedia - version of the 7/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
