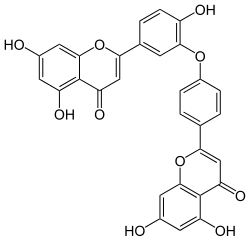
Ochnaflavone
 | |
| Names | |
|---|---|
| IUPAC name
2-[4-[5-(5,7-dihydroxy-4-oxochromen-2-yl)-2-hydroxyphenoxy]phenyl]-5,7-dihydroxychromen-4-one | |
| Identifiers | |
| 50276-96-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4590862 |
| PubChem | 5492110 |
| |
| |
| Properties | |
| C30H18O10 | |
| Molar mass | 538.46 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ochnaflavone, a secondary plant secondary metabolite of the Biflavonoid family, has been widely investigated in past decades due to its unique ability to mediate biological activities, such as inhibition of phospholipase A2 and lymphocyte proliferation. It was first isolated from Ochna squarrosa Linn, a member of Ochnaceae family, in 1973.[1]
Synthesis
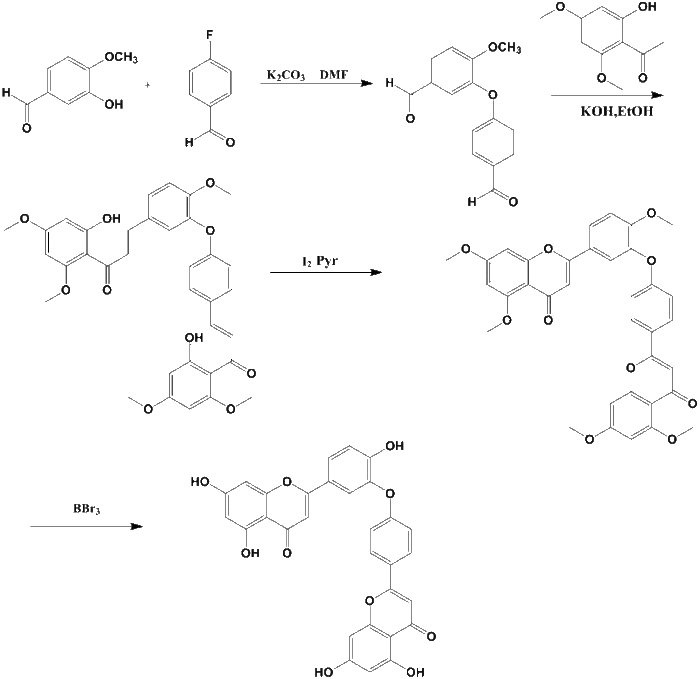
The first total synthesis of ochnaflavone derivatives was achieved by Kawano group in 1973, followed by the Heerden group for the first time reporting the total synthesis the ochnaflavone by constructing a diaryl ether intermediate and assemble the two flavone nuclei through ring cyclization.[2] To date, the field of biochemistry of biflavonoids is still wide open. Little is known about Ochnaflavone of its natural occurrence and biosythesis.[3]
Activity
Although Ochnaflavone has not been fully studied in the past decades, its protective role in plants and human body already attract scientists’ much attention, such as anti-atherogenic activity and anti-inflammatory activity.
Anti-atherogenic activity: Atherogenesis refers to the formation of atheromas on arteries’ walls. It is believed that atherogenesis may be caused by an infection of the vascular smooth muscle cells. Find molecules that possess anti-proliferation activity of vascular smooth muscle cells seems a good strategy to treat Atherogenesis. In 2006, a research group from Korea found Ochnaflavone could inhibit tumor necrosis factor (TNF)-a-induced human vascular smooth muscle cells proliferation. It was achieved by the ability of Ochnaflavone to regulate the extracellular signal-regulated kinase 1/2, matrix metalloproteinase-9 and cell cycle.[4]
Anti-inflammatory activity: Inflammation is a biological response to certain harmful stimuli. Phospholipase A2 (PLA2) is important in many inflammatory processes as it catalyses the release of arachidonic acid, which could generate lipid mediators of inflammation. Ochnaflavone is found to inhibit PLA2 by directly binding this enzyme irreversibly.[5]
References
- ↑ Okigawa, M.; Kawano, N.; Aqil, M.; Rahman, W. "The structure of ochnaflavone, a new type of biflavone and the synthesis of its pentamethyl ether". Tetrahedron Letters. 14 (22): 2003–2006. doi:10.1016/S0040-4039(01)96104-0.
- ↑ Ndoile, M. M.; van Heerden, F. R., Total synthesis of ochnaflavone. Beilstein Journal of Organic Chemistry 2013, 9, 1346–1351.
- ↑ Dey, P. M., METHODS IN PLANT BIOCHEMISTRY VOL 1 APL. Elsevier Science: 2012.
- ↑ Suh, S.-J.; Jin, U.-H.; Kim, S.-H.; Chang, H.-W.; Son, J.-K.; Ho Lee, S.; Son, K.-H.; Kim, C.-H., Ochnaflavone inhibits TNF-α-induced human VSMC proliferation via regulation of cell cycle, ERK1/2, and MMP-9. Journal of Cellular Biochemistry 2006, 99, 1298–1307.
- ↑ Chang, H. W.; Baek, S. H.; Chung, K. W.; Son, K. H.; Kim, H. P.; Kang, S. S., Inactivation of Phospholipase A2 by Naturally Occurring Biflavonoid, Ochnaflavone. Biochemical and Biophysical Research Communications 1994, 205, 843–849.