Occludin
| OCLN | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||
| |||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | OCLN, BLCPMG, PPP1R115, occludin | ||||||||||||||||
| External IDs | OMIM: 602876 MGI: 106183 HomoloGene: 1905 GeneCards: OCLN | ||||||||||||||||
| |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl |
|
||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 5: 69.49 – 69.56 Mb | Chr 13: 100.5 – 100.55 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
Occludin is a protein that in humans is encoded by the OCLN gene.[3][4] Occludin is a 65-kDa (522-amino acid polypeptide -human) integral plasma-membrane protein located at the tight junctions, described for the first time in 1993 by Shoichiro Tsukita.[5] Together with the Claudin group of proteins, it is the main component of the tight junctions.
Gene location
The OCLN gene is located on the long (q) arm of chromosome 5 at position q13.1. The gene starts at base pair 69,492,292 and goes to base pair 69,558,104 and is 65,813 base pairs long.[6]
Protein structure
Occludin's structure can be broken down into 9 domains. These domains are separated into two groups. 5 of the domains are located intracellularly and extracellularly. These 5 domains are separated by the 4 transmembrane domains of the protein. The nine domains are as follows:
- N-terminus domain (66 aa)
- transmembrane domain 1 (23 aa)
- extracellular loop 1 (46 aa)
- transmembrane domain 2 (25 aa)
- intracellular loop (10 aa)
- transmembrane domain 3 (25 aa)
- extracellular domain 2 (48 aa)
- transmembrane domain 4 (22 aa)
- C-terminus domain (257 aa)
The C-terminus domain has been shown experimentally to be required for correct assembly of tight junction barrier function.[7] The C-terminus also interacts with several cytoplasmic proteins of the junctional plaque and interacts with signaling molecules responsible for cell survival.[8] The N-terminus of occludin experimentally has been linked to involvement in tight junction sealing/barrier properties.[8] The extracellular loops are thought to be involved in the regulation of paracellualr permeability and the second extracellular has been shown to be involved in the localization of occludin at the tight junction.[8]
Function
Occludin is an important protein in tight junction function. Studies have shown that rather than being important in tight junction assembly, occludin is important in tight junction stability and barrier function. Furthermore, studies in which mice were deprived of occludin expression showed morphological stability in several epithelial tissues but also found chronic inflammation and hyperplasia in the gastric epithelium, calcification in the brain, testicular atrophy, loss of cytoplasmic granules in straited duct cells of salivary gland, and thinning of the compact bone. The phenotypical response of these mice to the lack of occludin suggest that the function of occludin is more complex than thought and requires more work.[9]
Role in cancer
Occludin plays a critical role in maintaining the barrier properties of a tight junction. Thus, mutation or absence of occludin increases epithelial leakiness which is an important barrier in preventing metastasis of cancer. Loss of occludin or abnormal expression of occludin has been shown to cause increased invasion, reduced adhesion and significantly reduced tight junction function in breast cancer tissues. Furthermore, patients with metastatic disease displayed significantly lower levels of occludin suggesting that the loss of occludin and thereby loss of tight junction integrity is important in metastatic development of breast cancer.[10]
Occludin also plays an important role in the apoptosis. The C-terminus of occludin is important in receiving and transmitting cell survival signals. In standard cells, loss or disruption of occludin and other tight junction proteins leads to initiation of apoptosis through extrinsic pathways.[11] Studies involving high levels of expression of occludin in cancer cells have shown that occludin mitigates several important cancer proliferation properties. The presence of occludin decreased cellular invasiveness and motility, enhanced cellular sensitivity to apoptogenic factors and lowered tumorigenesis and metastasis of the cancer cells. Specifically, occludin has a strong inhibitory effect on Raf1-induced tumorigenesis. Still, the exact mechanism of how occludin prevents the progression of cancer is not known but it has been shown that cancer progression is linked to the loss of occludin or the silencing of the OCLN gene.[12]
Disease linkage
Disruption of occludin regulation is an important aspect of a number of diseases. Strategies to prevent and/or reverse occludin downregulation may be an important therapeutic target. Mutation of occludin are thought to be a cause of band-like calcification with simple gyration and polymicrogyria (BLC-PMG). BLC-PMG is an autosomal recessive neurologic disorder.
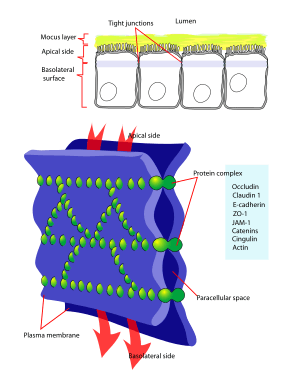
Interactions
Occludin has been shown to interact with Tight junction protein 2,[13][14][15] YES1[16] and Tight junction protein 1.[17][18]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Ando-Akatsuka Y, Saitou M, Hirase T, Kishi M, Sakakibara A, Itoh M, Yonemura S, Furuse M, Tsukita S (May 1996). "Interspecies diversity of the occludin sequence: cDNA cloning of human, mouse, dog, and rat-kangaroo homologues". J Cell Biol. 133 (1): 43–47. doi:10.1083/jcb.133.1.43. PMC 2120780
 . PMID 8601611.
. PMID 8601611. - ↑ "Entrez Gene: OCLN occludin".
- ↑ Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S (1993). "Occludin: a novel integral membrane protein localizing at tight junctions". J. Cell Biol. 123 (6 Pt 2): 1777–1788. doi:10.1083/jcb.123.6.1777. PMC 2290891
 . PMID 8276896.
. PMID 8276896. - ↑ http://www.ncbi.nlm.nih.gov/gene/100506658
- ↑ Chen Y, Merzdorf C, Paul DL, Goodenough DA (1997). "COOH terminus of occludin is required for tight junction barrier function in early Xenopus embryos". J. Cell Biol. 138: 891–899. doi:10.1083/jcb.138.4.891.
- 1 2 3 Feldman, Gemma J., James M. Mullin, and Michael P. Ryan. "Occludin: structure, function and regulation." Advanced drug delivery reviews 57.6 (2005): 883-917.
- ↑ Saitou M.; et al. (2000). "Complex phenotype of micelacking occludin, a component of tight junctionstrands". Mol. Biol. Cell. 11: 4131–4142.
- ↑ Martin, Tracey A., Robert E. Mansel, and Wen G. Jiang. "Loss of occludin leads to the progression of human breast cancer." International journal of molecular medicine 26.5 (2010): 723-734.
- ↑ Beeman, N., P. G. Webb, and H. K. Baumgartner. "Occludin is required for apoptosis when claudin–claudin interactions are disrupted." Cell death & disease 3.2 (2012): e273.
- ↑ Osanai, Makoto, et al. "Epigenetic silencing of occludin promotes tumorigenic and metastatic properties of cancer cells via modulations of unique sets of apoptosis-associated genes." Cancer research 66.18 (2006): 9125-9133.
- ↑ Peng, Bi-Hung; Lee J Ching; Campbell Gerald A (Dec 2003). "In vitro protein complex formation with cytoskeleton-anchoring domain of occludin identified by limited proteolysis". J. Biol. Chem. United States. 278 (49): 49644–49651. doi:10.1074/jbc.M302782200. ISSN 0021-9258. PMID 14512431.
- ↑ Itoh, M; Morita K; Tsukita S (Feb 1999). "Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin". J. Biol. Chem. UNITED STATES. 274 (9): 5981–5986. doi:10.1074/jbc.274.9.5981. ISSN 0021-9258. PMID 10026224.
- ↑ Wittchen, E S; Haskins J; Stevenson B R (Dec 1999). "Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3". J. Biol. Chem. UNITED STATES. 274 (49): 35179–35185. doi:10.1074/jbc.274.49.35179. ISSN 0021-9258. PMID 10575001.
- ↑ Chen, Yan-Hua; Lu Qun; Goodenough Daniel A; Jeansonne Beverly (Apr 2002). "Nonreceptor tyrosine kinase c-Yes interacts with occludin during tight junction formation in canine kidney epithelial cells". Mol. Biol. Cell. United States. 13 (4): 1227–1237. doi:10.1091/mbc.01-08-0423. ISSN 1059-1524. PMC 102264
 . PMID 11950934.
. PMID 11950934. - ↑ Fanning, A S; Jameson B J; Jesaitis L A; Anderson J M (Nov 1998). "The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton". J. Biol. Chem. UNITED STATES. 273 (45): 29745–29753. doi:10.1074/jbc.273.45.29745. ISSN 0021-9258. PMID 9792688.
- ↑ Rao, Radhakrishna K; Basuroy Shyamali, Rao Vijay U, Karnaky Jr Karl J, Gupta Akshay (Dec 2002). "Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress". Biochem. J. England. 368 (Pt 2): 471–81. doi:10.1042/BJ20011804. ISSN 0264-6021. PMC 1222996
 . PMID 12169098. Cite uses deprecated parameter
. PMID 12169098. Cite uses deprecated parameter |coauthors=(help)
Further reading
- Furuse M, Itoh M, Hirase T, et al. (1994). "Direct association of occludin with ZO-1 and its possible involvement in the localization of occludin at tight junctions". J. Cell Biol. 127 (6 Pt 1): 1617–1626. doi:10.1083/jcb.127.6.1617. PMC 2120300
 . PMID 7798316.
. PMID 7798316. - Van Itallie CM, Anderson JM (1997). "Occludin confers adhesiveness when expressed in fibroblasts". J. Cell. Sci. 110 (9): 1113–21. PMID 9175707.
- Kimura Y, Shiozaki H, Hirao M, et al. (1997). "Expression of occludin, tight-junction-associated protein, in human digestive tract". Am. J. Pathol. 151 (1): 45–54. PMC 1857944
 . PMID 9212730.
. PMID 9212730. - Saitou M, Ando-Akatsuka Y, Itoh M, et al. (1997). "Mammalian occludin in epithelial cells: its expression and subcellular distribution". Eur. J. Cell Biol. 73 (3): 222–31. PMID 9243183.
- Haskins J, Gu L, Wittchen ES, et al. (1998). "ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin". J. Cell Biol. 141 (1): 199–208. doi:10.1083/jcb.141.1.199. PMC 2132714
 . PMID 9531559.
. PMID 9531559. - Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998). "The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton". J. Biol. Chem. 273 (45): 29745–29753. doi:10.1074/jbc.273.45.29745. PMID 9792688.
- Itoh M, Morita K, Tsukita S (1999). "Characterization of ZO-2 as a MAGUK family member associated with tight as well as adherens junctions with a binding affinity to occludin and alpha catenin". J. Biol. Chem. 274 (9): 5981–5986. doi:10.1074/jbc.274.9.5981. PMID 10026224.
- Jiang WG, Martin TA, Matsumoto K, et al. (1999). "Hepatocyte growth factor/scatter factor decreases the expression of occludin and transendothelial resistance (TER) and increases paracellular permeability in human vascular endothelial cells". J. Cell. Physiol. 181 (2): 319–329. doi:10.1002/(SICI)1097-4652(199911)181:2<319::AID-JCP14>3.0.CO;2-S. PMID 10497311.
- Wittchen ES, Haskins J, Stevenson BR (2000). "Protein interactions at the tight junction. Actin has multiple binding partners, and ZO-1 forms independent complexes with ZO-2 and ZO-3". J. Biol. Chem. 274 (49): 35179–35185. doi:10.1074/jbc.274.49.35179. PMID 10575001.
- Kojima T, Sawada N, Chiba H, et al. (2000). "Induction of tight junctions in human connexin 32 (hCx32)-transfected mouse hepatocytes: connexin 32 interacts with occludin". Biochem. Biophys. Res. Commun. 266 (1): 222–229. doi:10.1006/bbrc.1999.1778. PMID 10581193.
- Burns AR, Bowden RA, MacDonell SD, et al. (2000). "Analysis of tight junctions during neutrophil transendothelial migration". J. Cell. Sci. 113 (1): 45–57. PMID 10591624.
- Itoh M, Furuse M, Morita K, et al. (2000). "Direct binding of three tight junction-associated MAGUKs, ZO-1, ZO-2, and ZO-3, with the COOH termini of claudins". J. Cell Biol. 147 (6): 1351–1363. doi:10.1083/jcb.147.6.1351. PMC 2168087
 . PMID 10601346.
. PMID 10601346. - Singh U, Van Itallie CM, Mitic LL, et al. (2000). "CaCo-2 cells treated with Clostridium perfringens enterotoxin form multiple large complex species, one of which contains the tight junction protein occludin". J. Biol. Chem. 275 (24): 18407–18417. doi:10.1074/jbc.M001530200. PMID 10749869.
- Marzioni D, Banita M, Felici A, et al. (2001). "Expression of ZO-1 and occludin in normal human placenta and in hydatidiform moles". Mol. Hum. Reprod. 7 (3): 279–285. doi:10.1093/molehr/7.3.279. PMID 11228248.
- Andreeva AY, Krause E, Müller EC, et al. (2001). "Protein kinase C regulates the phosphorylation and cellular localization of occludin". J. Biol. Chem. 276 (42): 38480–38486. doi:10.1074/jbc.M104923200. PMID 11502742.
- Papadopoulos MC, Saadoun S, Woodrow CJ, et al. (2001). "Occludin expression in microvessels of neoplastic and non-neoplastic human brain". Neuropathol. Appl. Neurobiol. 27 (5): 384–395. doi:10.1046/j.0305-1846.2001.00341.x. PMID 11679090.
- Schmidt A, Utepbergenov DI, Krause G, Blasig IE (2001). "Use of surface plasmon resonance for real-time analysis of the interaction of ZO-1 and occludin". Biochem. Biophys. Res. Commun. 288 (5): 1194–1199. doi:10.1006/bbrc.2001.5914. PMID 11700038.
- Pummi K, Malminen M, Aho H, et al. (2001). "Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes". J. Invest. Dermatol. 117 (5): 1050–1058. doi:10.1046/j.0022-202x.2001.01493.x. PMID 11710912.
- Traweger A, Fang D, Liu YC, et al. (2002). "The tight junction-specific protein occludin is a functional target of the E3 ubiquitin-protein ligase itch". J. Biol. Chem. 277 (12): 10201–10208. doi:10.1074/jbc.M111384200. PMID 11782481.
External links
- Vivian Tang. "OCCLUDIN in Focus". www.Zonapse.Net. Retrieved 2008-02-10.
- Vivian Tang. "Tight Junction Overview". www.Zonapse.Net. Retrieved 2008-02-10.
- GeneTests/NCBI/NIH/UW entry on Band-Like Calcification with Simplified Gyration and Polymicrogyria

