Neutron capture

| Science with Neutrons |
|---|
 |
| Foundations |
| Neutron scattering |
| Other applications |
|
| Infrastructure |
|
| Neutron facilities |
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus.[1] Since neutrons have no electric charge they can enter a nucleus more easily than positively charged protons, which are repelled electrostatically.[1]
Neutron capture plays an important role in the cosmic nucleosynthesis of heavy elements. In stars it can proceed in two ways: as a rapid (r-process) or a slow process (s-process).[1] Nuclei of masses greater than 56 cannot be formed by thermonuclear reactions (i.e. by nuclear fusion), but can be formed by neutron capture.[1]
Neutron capture at small neutron flux
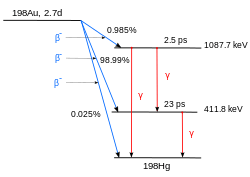
At small neutron flux, as in a nuclear reactor, a single neutron is captured by a nucleus. For example, when natural gold (197Au) is irradiated by neutrons, the isotope 198Au is formed in a highly excited state, and quickly decays to the ground state of 198Au by the emission of γ rays. In this process, the mass number increases by one. This is written as a formula in the form 197Au+n → 198Au+γ, or in short form 197Au(n,γ)198Au. If thermal neutrons are used, the process is called thermal capture.
The isotope 198Au is a beta emitter that decays into the mercury isotope 198Hg. In this process the atomic number rises by one.
Neutron capture at high neutron flux
The r-process happens inside stars if the neutron flux density is so high that the atomic nucleus has no time to decay via beta emission in between neutron captures. The mass number therefore rises by a large amount while the atomic number (i.e., the element) stays the same. Only afterwards, the highly unstable nuclei decay via many β− decays to stable or unstable nuclei of high atomic number.
Capture cross section
The absorption neutron cross-section of an isotope of a chemical element is the effective cross sectional area that an atom of that isotope presents to absorption, and is a measure of the probability of neutron capture. It is usually measured in barns (b).
Absorption cross section is often highly dependent on neutron energy. As a generality, the likelihood of absorption is proportional to the time the neutron is in the vicinity of the nucleus. The time spent in the vicinity of the nucleus is inversely proportional to the relative velocity between the neutron and nucleus. Other more specific issues modify this general principle. Two of the most commonly specified measures are the cross-section for thermal neutron absorption, and resonance integral which considers the contribution of absorption peaks at certain neutron energies specific to a particular nuclide, usually above the thermal range, but encountered as neutron moderation slows the neutron down from an original high energy.
The thermal energy of the nucleus also has an effect; as temperatures rise, Doppler broadening increases the chance of catching a resonance peak. In particular, the increase in uranium-238's ability to absorb neutrons at higher temperatures (and to do so without fissioning) is a negative feedback mechanism that helps keep nuclear reactors under control.
Thermochemical significance
Neutron capture is involved in the formation of isotopes of chemical elements. As a consequence of this fact the energy of neutron capture intervenes in the standard enthalpy of formation of isotopes.
Uses
Neutron activation analysis can be used to remotely detect the chemical composition of materials. This is because different elements release different characteristic radiation when they absorb neutrons. This makes it useful in many fields related to mineral exploration and security.
Neutron absorbers

The most important neutron absorber is 10B as 10B4C in control rods, or boric acid as a coolant water additive in PWRs. Other important neutron absorbers that are used in nuclear reactors are xenon, cadmium, hafnium, gadolinium, cobalt, samarium, titanium, dysprosium, erbium, europium, molybdenum and ytterbium;[2] all of which usually consist of mixtures of various isotopes—some of which are excellent neutron-absorbers. These also occur in combinations such as Mo2B5, hafnium diboride, titanium diboride, dysprosium titanate and gadolinium titanate.
Hafnium, one of the last stable elements to be discovered, presents an interesting case. Even though hafnium is a heavier element, its electron configuration makes it practically identical with the element zirconium, and they are always found in the same ores. However, their nuclear properties are different in a profound way. Hafnium absorbs neutrons avidly (Hf absorbs 600 times more than Zr), and it can be used in reactor control rods, whereas natural zirconium is practically transparent to neutrons. So, zirconium is a very desirable construction material for reactor internal parts, including the metallic cladding of the fuel rods which contain either uranium, plutonium, or mixed oxides of the two elements (MOX fuel).
Hence, it is quite important to be able to separate the zirconium from the hafnium in their naturally-occurring alloy. This can only be done inexpensively by using modern chemical ion-exchange resins.[3] Similar resins are also used in reprocessing nuclear fuel rods, when it is necessary to separate uranium and plutonium, and sometimes thorium.
See also
- Beta decay
- Induced radioactivity
- List of particles
- Neutron emission
- Radioactive decay
- Rays: α — β — γ — δ — ε
- p-process (proton capture)
References
- 1 2 3 4 Ahmad, Ishfaq; Hans Mes; Jacques Hebert (1966). "Progress of theoretical physics: Resonance in the Nucleus". Institute of Physics. Ottawa, Canada: University of Ottawa (Department of Physics). 3 (3): 556–600.
- ↑ Prompt Gamma-ray Neutron Activation Analysis. International Atomic Energy Agency
- ↑ D. Franklin; R. B. Adamson (1 January 1984). Zirconium in the Nuclear Industry: Sixth International Symposium. ASTM International. pp. 26–. ISBN 978-0-8031-0270-5. Retrieved 7 October 2012.
External links
- Thermal Neutron Capture Data
- Thermal Neutron Cross Sections at the International Atomic Energy Agency