Neural tube defect
| Neural tube defect | |
|---|---|
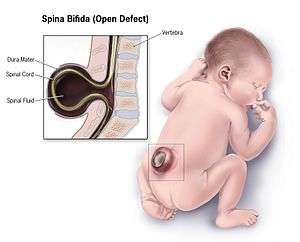 | |
| Illustration of a child with spina bifida the most common NTD | |
| Classification and external resources | |
| Specialty | medical genetics |
| ICD-10 | Q00, Q01, Q05 |
| ICD-9-CM | 740, 741, 742 |
| OMIM | 182940 301410 |
| DiseasesDB | 8926 |
| eMedicine | neuro/244 ped/2805 |
| MeSH | D009436 |
Neural tube defects (NTDs) are a group of conditions in which an opening in the spinal cord or brain remains from early in human development. In the 3rd week of pregnancy called gastrulation, specialized cells on the dorsal side of the embryo begin to change shape and form the neural tube. When the neural tube does not close completely, an NTD develops.
Specific types include: spina bifida which affects the spine, anencephaly which results in little to no brain, encephalocele which affects the skull, and iniencephaly which results in severe neck problems.[1]
NTDs are one of the most common birth defects, affecting over 300,000 births each year worldwide.[2] For example, spina bifida affects approximately 1,500 births annually in the USA, or about 3.5 in every 10,000 (0.035% of US births),[3][4] which has decreased from around 5 per 10,000 (0.05% of US births) since folate fortification was started.[4] The number of deaths in the USA each year due to neural tube defects also declined from 1,200 before folate fortification was started to 840.[5]
Types
There are two types of NTDs: open, which are more common, and closed. Open NTDs occur when the brain and/or spinal cord are exposed at birth through a defect in the skull or vertebrae (back bones). Examples of open NTDs are anencephaly, encephaloceles, hydranencephaly, iniencephaly, schizencephaly, and spina bifida. Rarer types of NTDs are called closed NTDs. Closed NTDs occur when the spinal defect is covered by skin. Common examples of closed NTDs are lipomyelomeningocele, lipomeningocele, and tethered cord.
Anencephaly
Anencephaly (without brain) is a neural tube defect that occurs when the head end of the neural tube fails to close, usually during the 23rd and 26th days of pregnancy, resulting in an absence of a major portion of the brain and skull. Infants born with this condition are born without the main part of the forebrain—the largest part of the cerebrum—and are usually blind, deaf and unconscious. The lack of a functioning cerebrum will ensure that the infant will never gain consciousness. Infants are either stillborn or usually die within a few hours or days after birth.
Encephaloceles
Encephaloceles are characterized by protrusions of the brain through the skull that are sac-like and covered with membrane. They can be a groove down the middle of the upper part of the skull, between the forehead and nose, or the back of the skull. Encephaloceles are often obvious and diagnosed immediately. Sometimes small encephaloceles in the nasal and forehead are undetected. http://www.ninds.nih.gov/disorders/encephaloceles/encephaloceles.htm
Hydranencephaly
Hydranencephaly is a condition in which the cerebral hemispheres are missing and instead filled with sacs of cerebrospinal fluid.
Iniencephaly
Iniencephaly is a rare neural tube defect that results in extreme bending of the head to the spine. The diagnosis can usually be made on antenatal ultrasound scanning, but if not will undoubtedly be made immediately after birth because the head is bent backwards and the face looks upwards. Usually the neck is absent. The skin of the face connects directly to the chest and the scalp connects to the upper back. The infant will usually not survive more than a few hours.
Spina bifida
Spina bifida is further divided into two subclasses, spina bifida cystica and spina bifida occulta.
Spina bifida cystica
This includes meningocele and myelomeningocele. Meningocele is less severe and is characterized by herniation of the meninges, but not the spinal cord, through the opening in the spinal canal. Myelomeningocele involves herniation of the meninges as well as the spinal cord through the opening.[6]
Spina bifida occulta
In this type of neural tube defect, the meninges do not herniate through the opening in the spinal canal.[6] It is a common condition, occurring in 10–20% of otherwise healthy people. By definition, spina bifida occulta means hidden split spine.[7] The most frequently seen form of spina bifida occulta is when parts of the bones of the spine, called the spinous process, and the neural arch appear abnormal on a radiogram, and is generally harmless. Usually the spinal cord and spinal nerves are not involved.[8] The risk of recurrence in those who have a first degree relative (e.g. parent, sibling) is 5–10 times greater than that in the general population. The genetic risk of recurrence with symptomatic forms of spina bifida occulta is uncertain.
Cause
Folate deficiency
Folate (vitamin B9) and vitamin B12 are very important in reducing the occurrences of NTDs.[9] Folate is required for the production and maintenance of new cells, for DNA synthesis and RNA synthesis. Folate is needed to carry one carbon groups for methylation and nucleic acid synthesis. It has been hypothesized that the early human embryo may be particularly vulnerable to folate deficiency due to differences of the functional enzymes in this pathway during embryogenesis combined with high demand for post translational methylations of the cytoskeleton in neural cells during neural tube closure.[10] Failure of post-translational methylation of the cytoskeleton, required for differentiation has been implicated in neural tube defects.[11] Vitamin B12 is also an important receptor in the folate biopathway such that studies have shown deficiency in vitamin B12 contributes to risk of NTDs as well.[12] Importantly, a deficiency of folate itself does not cause neural tube defects. The association seen between reduced neural tube defects and folic acid supplementation is due to a gene-environment interaction such as venerability caused by the C677T Methylenetetrahydrofolate reductase (MTHFR) variant. Supplementing folic acid during pregnancy reduces the prevalence of NTDs by not exposing this otherwise sub-clinical mutation to aggravating conditions.[13] There is substantial evidence that direct folic supplementation increases blood serum levels of bioavailable folate even though at least one study have shown slow and variable activity of dihydrofolate reductase in human liver.[14][15] A diet rich in natural folate (350 μg/d) can show as much increase in plasma folate as taking low levels of folic acid (250 μg/d) in individuals[16] However a comparison of general population outcomes across many countries with different approaches to increasing folate consumption has found that only general food fortification with folic acid reduces neural tube defects [17] While there have been concerns about folic acid supplementation being linked to an increased risk for cancer, a systematic review in 2012 shows there is no evidence except in the case of prostate cancer which indicates a modest reduction in risk.[18]
Gene-environment interaction
Other potential causes can include folate antimetabolites (such as methotrexate), maternal diabetes, maternal obesity, mycotoxins in contaminated corn meal, arsenic, hyperthermia in early development, and radiation.[19][20][21] Studies have shown that both maternal cigarette smoking and maternal exposure to secondhand smoke increased the risk for neural tube defects in offspring.[22] A mechanism by which maternal exposure to cigarette smoke could increase NTD risk in offspring is suggested by several studies that show an association between cigarette smoking and elevations of homocysteine levels. The study suggests that cigarette smoke, including secondhand exposure, is not only hazardous to the mother, but may also interfere with neural tube closure in the developing embryo.[23] All of the above may act by interference with some aspect of normal folic acid metabolism and folate linked methylation related cellular processes as there are multiple genes of this type associated with neural tube defects.[24]
Other
Folic acid supplementation reduces the prevalence of neural tube defects by approximately 70% of neural tube defects indicating that 30% are not folate-dependent and are due to some cause other than alterations of methylation patterns.[25] Multiple other genes related to neural tube defects exist which are candidates for folate insensitive neural tube defects.[24] There are also several syndromes such as Meckel syndrome, and Triploid Syndrome which are frequently accompanied by neural tube defects that are assumed to be unrelated to folate metabolism[26]
Diagnosis
Tests for neural tube defects include ultrasound examination and measurement of maternal serum alpha-fetoprotein (MSAFP). Amniotic fluid alpha-fetoprotein (AFAFP) and amniotic fluid acetylcholinesterase (AFAChE) tests are also used to confirming if ultrasound screening indicates a positive risk.[27] Often, these defects are apparent at birth, but acute defects may not be diagnosed until much later in life. An elevated MSAFP measured at 16–18 weeks gestation is a good predictor of open neural tube defects, however the test has a very high false positive rate, (2% of all women tested in Ontario, Canada between 1993 and 2000 tested positive without having an open neural tube defect, although 5% is the commonly quoted result worldwide) and only a portion of neural tube defects are detected by this screen test (73% in the same Ontario study).[28] MSAFP screening combined with routine ultrasonography has the best detection rate although detection by ultrasonography is dependent on operator training and the quality of the equipment.[29][30]
Prevention
In 1996, the United States Food and Drug Administration published regulations requiring the addition of folic acid to enriched breads, cereals, flour and other grain products.[31] It is important to note that during the first four weeks of pregnancy (when most women do not even realize that they are pregnant), adequate folate intake is essential for proper operation of the neurulation process. Therefore, women who could become pregnant are advised to eat foods fortified with folic acid or take supplements in addition to eating folate-rich foods to reduce the risks of serious birth defects.[32][33][34] In Canada, mandatory fortification of selected foods with folic acid has been shown to reduce the incidence of neural tube defects by 46%.[35]
Women who may become pregnant are advised to get 400 micrograms of folic acid daily. Women who are pregnant should receive 1.0 mg (1000 mcg), and women who have previously given birth to a child with a neural tube defect should get 4.0 mg/5.0 mg in the UK mg daily.[36]
Treatment
Treatments of NTDs depends on the severity of the complication. No treatment is available for anencephaly and infants usually do not survive more than a few hours. Aggressive surgical management has improved survival and the functions of infants with spina bifida, meningoceles and mild myelomeningoceles. The success of surgery often depends on the amount of brain tissue involved in the encephalocele. The goal of treatment for NTDs is to allow the individual to achieve the highest level of function and independence. Fetal surgery in utero before 26 weeks gestation has been performed with some hope that there is benefit to the final outcome including a reduction in Arnold–Chiari malformation and thereby decreases the need for a ventriculoperitoneal shunt but the procedure is very high risk for both mother and baby and is considered extremely invasive with questions that the positive outcomes may be due to ascertainment bias and not true benefit. Further, this surgery is not a cure for all problems associated with a neural tube defect. Other areas of research include tissue engineering and stem cell therapy but this research has not been used in humans.[37]
Epidemiology
Neural tube defects resulted in 71,000 deaths globally in 2010.[38]
References
- ↑ "Neural Tube Defects (NTDs): Condition Information". 2012-11-30. Retrieved 8 May 2015.
- ↑ National Center on Birth Defects and Developmental Disabilities (2012). "Neural Tube Defects (Annual Report)" (PDF). US Centers for Disease Control and Prevention.
- ↑ National Institute of Child Health and Human Development (30 November 2012). "How many people are affected by or are at risk for neural tube defects?". Neural Tube Defects (NTDs). U.S. National Institutes of Health. External link in
|work=(help) - 1 2 National Center on Birth Defects and Developmental Disabilities. "Spina Bifida - Data and Statistics". US Centers for Disease Control and Prevention. Retrieved 17 January 2014.
- ↑ National Center on Birth Defects and Developmental Disabilities. "Folic Acid—Birth Defects COUNT". US Centers for Disease Control and Prevention. Retrieved 13 May 2014.
- 1 2 Le, Tao; Bhushan, Vikas; Vasan, Neil (2010). First Aid for the USMLE Step 1: 2010 (20th ed.). McGraw-Hill. p. 127. ISBN 978-0-07-163340-6.
- ↑ Saladin, Kenneth (2010). Anatomy and Physiology: The Unity of Form and Function. McGraw-Hill. p. 485. ISBN 978-0-07-352569-3.
- ↑ Pittman, T (2008). "Spina bifida occulta.". Journal of Neurosurgery. Pediatrics. 1 (2): 113; discussion 113. doi:10.3171/PED/2008/1/2/113. PMID 18352777.
- ↑ Molloy, A. M.; Kirke, P. N.; Troendle, J. F.; Burke, H.; Sutton, M.; Brody, L. C.; Scott, JM; Mills, JL (2009). "Maternal Vitamin B-12 Status and Risk of Neural Tube Defects in a Population With High Neural Tube Defect Prevalence and No Folic Acid Fortification". Pediatrics. 123 (3): 917–923. doi:10.1542/peds.2008-1173. PMID 19255021.
- ↑ Bjorklund N, Gordon R(2006) A hypothesis linking low folate intake to neural tube defects due to failure of post-translation methylations of the cytoskeleton Int. J. Dev. Biol. 50: 135 - 141 doi: 10.1387/ijdb.052102nb
- ↑ Akchiche; et al. (2012). "Homocysteinylation of neuronal proteins contributes to folate deficiency-associated alterations of differentiation, vesicular transport, and plasticity in hippocampal neuronal cells". The FASEB Journal. 26 (10): 3980–3992. doi:10.1096/fj.12-205757.
- ↑ Li, F.; Watkins, D.; Rosenblatt, D. S. (2009). "Vitamin B-12 and birth defects". Molecular Genetics and Metabolism. 98 (1–2): 166–172. doi:10.1016/j.ymgme.2009.06.004. PMID 19586788.
- ↑ Yan, L; Zhao, L; Long, Y; Zou, P; Ji, G; Gu, A; Zhao, P (October 3, 2012). "Association of the Maternal MTHFR C677T Polymorphism with Susceptibility to Neural Tube Defects in Offsprings: Evidence from 25 Case-Control Studies". PLOS One. 7: e41689. doi:10.1371/journal.pone.0041689.

- ↑ Anderson C.; et al. "Response of serum and red blood cell folate concentrations to folic acid supplementation depends on methylenetetrahydrofolate reductase C677T genotype: results from a crossover trial". Mol. Nutr. Food Res. 57: 637–644. doi:10.1002/mnfr.201200108.
- ↑ Bailey SW, Ayling JE (Sep 2009). "The extremely slow and variable activity of dihydrofolate reductase in human liver and its implications for high folic acid intake". Proc Natl Acad Sci U S A. 106 (36): 15424–9. doi:10.1073/pnas.0902072106. PMC 2730961
 . PMID 19706381.
. PMID 19706381. - ↑ "Dietary Folate from Vegetables and Citrus Fruit Decreases Plasma Homocysteine Concentrations in Humans in a Dietary Controlled Trial". J. Nutr. 129 (6): 1135–1139. 1999.
- ↑ International retrospective cohort study of neural tube defects in relation to folic acid recommendations: are the recommendations working? BMJ 2005;330:571
- ↑ Wein, TN; Pike, E; Wisløff, T; Staff, A; Smeland, S; Klemp, M (12 January 2012). "Cancer risk with folic acid supplements: a systematic review and meta-analysis". BMJ Open. 2 (1): e000653. doi:10.1136/bmjopen-2011-000653. PMC 3278486
 . PMID 22240654.
. PMID 22240654. 
- ↑ Neural Tube Defects at eMedicine
- ↑ Suarez, L.; Brender, J. D.; Langlois, P. H.; Zhan, F. B.; Moody, K. (2007). "Pregnant women taking medication for epilepsy have a higher chance of having a child with a neural tube defect. Maternal exposures to hazardous waste sites and industrial facilities and risk of neural tube defects in offspring". Annals of Epidemiology. 17 (10): 772–7. doi:10.1016/j.annepidem.2007.05.005. PMID 17689262.
- ↑ Zhou, F. C.; Fang, Y.; Goodlett, C. (2008). "Peptidergic Agonists of Activity-Dependent Neurotrophic Factor Protect Against Prenatal Alcohol-Induced Neural Tube Defects and Serotonin Neuron Loss". Alcoholism: Clinical and Experimental Research. 32 (8): 1361–71. doi:10.1111/j.1530-0277.2008.00722.x. PMC 2758042
 . PMID 18565153.
. PMID 18565153. - ↑ Wang, M; Wang, ZP; Gong, R; Zhao, ZT (January 2014). "Maternal smoking during pregnancy and neural tube defects in offspring: a meta-analysis.". Child's Nervous System. 30 (1): 83–9. doi:10.1007/s00381-013-2194-5. PMID 23760473.
- ↑ Suarez, L.; Felkner, M.; Brender, J. D.; Canfield, M.; Hendricks, K. (2008). "Maternal exposures to cigarette smoke, alcohol, and street drugs and neural tube defect occurrence in offspring". Maternal and Child Health Journal. 12 (3): 394–401. doi:10.1007/s10995-007-0251-y. PMID 17641961.
- 1 2 Genetics of human neural tube defects Hum. Mol. Genet. (2009) 18 (R2): R113-R129. doi: 10.1093/hmg/ddp347
- ↑ Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991 Jul 20;338(8760):131-7.
- ↑ Rose, N, Mennuti, M, Glob. Fetal Neural Tube Defects: Diagnosis, Management, and Treatment libr. women's med., (ISSN 1756-2228) 2009; DOI 10.3843/GLOWM.10224
- ↑ Milunsky A, Alpert E (1984). "Results and benefits of a maternal serum alpha-fetoprotein screening program". JAMA. 252 (11): 1438–42. doi:10.1001/jama.252.11.1438. PMID 6206249.
- ↑ Summer Maternal Serum Screening in Ontario Using the Triple Marker Test J Med Screen September 2003 vol. 10 no. 3 107-111.
- ↑ Boyd et.al Survey of prenatal screening policies in Europe for structural malformations and chromosome anomalies, and their impact on detection and termination rates for neural tube defects and Down’s syndrome BJOG: An International Journal of Obstetrics & Gynaecology Volume 115, Issue 6, pages 689–696, May 2008
- ↑ Norem et.al Routine Ultrasonography Compared With Maternal Serum Alpha-fetoprotein for Neural Tube Defect Screening Obstetrics & Gynecology: October 2005 Vol 106:4 pp 747-52
- ↑ Daly S, Mills JL, Molloy AM, Conley M, Lee YJ, Kirke PN, Weir DG, Scott JM (1997). "Minimum effective dose of folic acid for food fortification to prevent neural-tube defects". Lancet. 350 (9092): 1666–9. doi:10.1016/S0140-6736(97)07247-4. PMID 9400511.
- ↑ Greene, ND; Stanier, P; Copp, AJ (2009). "Genetics of human neural tube defects". Human Molecular Genetics. 18 (R2): R113–29. doi:10.1093/hmg/ddp347. PMC 2758708
 . PMID 19808787.
. PMID 19808787. - ↑ Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ, Willett W (1989). "Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects". Journal of the American Medical Association. 262 (20): 2847–52. doi:10.1001/jama.262.20.2847. PMID 2478730.
- ↑ Goh, YI; Koren, G (2008). "Folic acid in pregnancy and fetal outcomes". J. Obstet. Gynaecol. 28 (1): 3–13. doi:10.1080/01443610701814195. PMID 18259891.
- ↑ De Wals P, Tairou F, Van Allen MI, et al. (2007). "Reduction in neural-tube defects after folic acid fortification in Canada". N Engl J Med. 357 (2): 135–142. doi:10.1056/NEJMoa067103. PMID 17625125.
- ↑ Centers for Disease Control (11 September 1992). "Recommendations for the Use of Folic Acid to Reduce the Number of Cases of Spina Bifida and Other Neural Tube Defects". Morbidity and Mortality Weekly Report. 41 (RR-14): 001.
- ↑ Sutton, LM Fetal surgery for neural tube defects Best Practice & Research Clinical Obstetrics & Gynaecology Volume 22, Issue 1, February 2008, Pages 175–188
- ↑ Lozano, R (Dec 15, 2012). "Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010.". Lancet. 380 (9859): 2095–128. doi:10.1016/S0140-6736(12)61728-0. PMID 23245604.
External links
- "Neural Tube Defects". MedlinePlus. U.S. National Library of Medicine.
- St. Joseph's Hospital and Medical Center Fetal Care Center
- Preventing Neural Tube Birth Defects: A Prevention Model and Resource Guide: Centre for Disease Control and Prevention (CDC)