Nepicastat
 | |
| Names | |
|---|---|
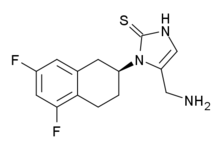
| IUPAC name
5-(aminomethyl)-1-[(2S)-5,7-difluoro-1,2,3,4-tetrahydronaphthalen-2-yl]-1,3-dihydro-2H-imidazole-2-thione | |
| Other names
SYN-117 | |
| Identifiers | |
| 173997-05-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 7971947 |
| 6630 | |
| MeSH | Nepicastat |
| PubChem | 9796181 |
| UNII | VPG12K4540 |
| |
| |
| Properties | |
| C14H15F2N3S | |
| Molar mass | 295.35 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Nepicastat (INN, codenamed SYN117, RS-25560-197) is an inhibitor of dopamine beta-hydroxylase, an enzyme that catalyzes the conversion of dopamine to norepinephrine.[1]
It has been studied as a possible treatment for congestive heart failure, and appears to be well tolerated as such.[2] As of 2012, clinical trials to assess nepicastat as a treatment for post-traumatic stress disorder (PTSD) and cocaine dependence have been completed.[3][4] In Phase 2 study treatment with nepicastat was not effective in relieving PTSD-associated symptoms when compared to placebo. The study was funded by the U.S. Department of Defense.[5]
References
- ↑ Stanley WC, Li B, Bonhaus DW, et al. (August 1997). "Catecholamine modulatory effects of nepicastat (RS-25560-197), a novel, potent and selective inhibitor of dopamine-beta-hydroxylase". Br J Pharmacol. 121 (8): 1803–9. doi:10.1038/sj.bjp.0701315. PMC 1564872
 . PMID 9283721.
. PMID 9283721. - ↑ Hegde SS, Friday KF (December 1998). "Dopamine-beta-hydroxylase inhibition: a novel sympatho-modulatory approach for the treatment of congestive heart failure". Current pharmaceutical design. 4 (6): 469–79. PMID 10197057.
- ↑ "Pharmacogenetic Clinical Trial of Nepicastat for Post Traumatic Stress Disorder (PTSD)". ClinicalTrials.gov. U.S. National Institutes of Health. June 4, 2008. Retrieved on February 1, 2012.
- ↑ "Study of Safety and Potential Efficacy of SYN117 in Cocaine Dependent Volunteers". ClinicalTrials.gov. U.S. National Institutes of Health. August 15, 2008. Retrieved on February 1, 2012.
- ↑ Biotie reports top-line data from clinical study with nepicastat (SYN117) in post-traumatic stress disorder BIOTIE THERAPIES CORP. STOCK EXCHANGE RELEASE 27 December 2012.
This article is issued from Wikipedia - version of the 9/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.