Multistriatin
 | |
| Names | |
|---|---|
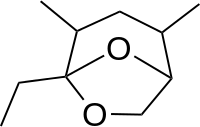
| IUPAC name
5-ethyl-2,4-dimethyl-6,8-dioxabicyclo[3.2.1]octane | |
| Other names
(1S,2R,4S,5R)-5-ethyl-2,4-dimethyl-6,8-dioxabicyclo[3.2.1]octane | |
| Identifiers | |
| 54815-06-4 | |
| 3D model (Jmol) | Interactive image |
| |
| Properties | |
| C10H18O2 | |
| Molar mass | 170.25 g·mol−1 |
| Density | 0.959 g/mL |
| Boiling point | 207.1 °C (404.8 °F; 480.2 K) |
| Hazards | |
| Flash point | 74.9 °C (166.8 °F; 348.0 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Multistriatin is a pheromone of the elm bark beetle. It is a volatile compound released by a virgin female beetle when she has found a good source of food, such as an elm tree.[1]
Potential applications
Males beetles, which carry the fungus which causes Dutch elm disease, are attracted to the pheromone. Hence multistriatin could be used to trap beetles and so prevent the spread of the disease.[1]
Stereochemistry
The compound exists in several diastereomic forms, depending on the positions of the methyl groups.[2] Only the natural stereoisomer, α-multistriatin, attracts the elm bark beetles.

References
- 1 2 Warren, Stuart (1982). Organic synthesis : the disconnection approach.
- ↑ Beck, Keith (1 September 1978). "Pheromone chemistry of the smaller European elm bark beetle". Journal of Chemical Education. 55 (9): 567. doi:10.1021/ed055p567.
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.