Biology of depression
Scientific studies have found that numerous brain areas show altered activity in patients suffering from depression, and this has encouraged advocates of various theories that seek to identify a biochemical origin of the disease, as opposed to theories that emphasize psychological or situational causes. Several theories concerning the biologically based cause of depression have been suggested over the years, of which the most prominent and widely researched is the monoamine hypothesis.
Genetic factors
In contrast to other psychiatric disorders such as autism and schizophrenia, genetic factors involved in depression have been more difficult to identify. In 2003 Science published an influential[1] study of Avshalom Caspi et al. who found that a gene-environment interaction (GxE) may explain why life stress is a predictor for depressive episodes in some individuals, but not in others, depending on an allelic variation of the serotonin-transporter-linked promoter region (5-HTTLPR).[2] Soon after, the results were replicated by Kenneth Kendler's group, raising hopes in the psychiatric genetics community.[3] By 2007 there were 11 replications, 3 partial replication and 3 non-replications of this proposed GxE. However, two of the largest studies[4][5] were negative.[6] Two 2009 meta-analyses were also negative; one included 14 studies,[7] the other just five, owing to different study selection criteria.[8] A 2010 review of studies in this area found 17 replications, 8 partial replications (interaction only in females or only with one of several types of adversity), and 9 non-replications (no interaction or an interaction in the opposite direction). It also found a systematic relationship between the method used to assess environmental adversity and the results of the studies; all studies using objective indicators or structured interviews to assess stress replicated the gene–environment interaction fully or partially, whereas all non-replications relied on brief self-report measures of adversity. This review also found that both 2009 meta-analyses were significantly biased toward negative studies.[9]
Other hypothesized genomic influences are BDNF polymorphisms, but the replications studies have been mixed and insufficient as of 2005 for a meta-analysis.[10] Studies also indicate an association of BDNF to suicidal behavior.[11] However, findings from the gene-environment interactions studies suggest that the current BDNF models of depression are too simplistic.[12] A 2008 study found interactions (biological epistasis) in the signaling pathways of the BDNF and the serotonin transporter; the BDNF Val66Met allele, which was predicted to have reduced responsitivity to serotonin, was found to exercise protective effects in individuals with the short 5-HTTLPR allele that is otherwise believed to predispose individuals to depressive episodes after stressful events.[13] Thus, the BDNF-mediated signalling involved in neuroplastic responses to stress and antidepressants is influenced by other genetic and environmental modifiers.[12]
The largest genome-wide study to date failed to identify variants with genome-wide significance in over 9000 cases.[14]
Recently, the first genetics study has been published with positively identified two variants with genome-wide association with major depressive disorder.[15] This study, conducted in Chinese Han woman, identified two variants in intronic regions near SIRT1 and LHPP.
Circadian rhythm
Depression may be related to abnormalities in the circadian rhythm,[16] or biological clock. For example, rapid eye movement (REM) sleep—the stage in which dreaming occurs—may be quick to arrive and intense in depressed people. REM sleep depends on decreased serotonin levels in the brain stem,[17] and is impaired by compounds, such as antidepressants, that increase serotonergic tone in brain stem structures.[17] Overall, the serotonergic system is least active during sleep and most active during wakefulness. Prolonged wakefulness due to sleep deprivation[16] activates serotonergic neurons, leading to processes similar to the therapeutic effect of antidepressants, such as the selective serotonin reuptake inhibitors (SSRIs). Depressed individuals can exhibit a significant lift in mood after a night of sleep deprivation. SSRIs may directly depend on the increase of central serotonergic neurotransmission for their therapeutic effect, the same system that impacts cycles of sleep and wakefulness.[17]
Research on the effects of light therapy on seasonal affective disorder suggests that light deprivation is related to decreased activity in the serotonergic system and to abnormalities in the sleep cycle, particularly insomnia. Exposure to light also targets the serotonergic system, providing more support for the important role this system may play in depression.[18] Sleep deprivation and light therapy both target the same brain neurotransmitter system and brain areas as antidepressant drugs, and are now used clinically to treat depression.[19] Light therapy, sleep deprivation and sleep time displacement (sleep phase advance therapy) are being used in combination quickly to interrupt a deep depression in hospitalized patients.[18]
Monoamines
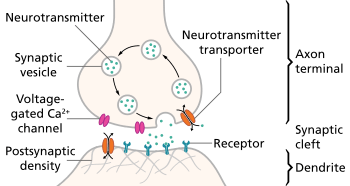
Monoamines are neurotransmitters that include serotonin, dopamine, norepinephrine, and epinephrine.[20] Many antidepressant drugs increase synaptic levels of the monoamine neurotransmitter, serotonin, but they may also enhance the levels of two other neurotransmitters, norepinephrine and dopamine. The observation of this efficacy led to the monoamine hypothesis of depression, which postulates that the deficit of certain neurotransmitters is responsible for the corresponding features of depression: "Norepinephrine may be related to alertness and energy as well as anxiety, attention, and interest in life; [lack of] serotonin to anxiety, obsessions, and compulsions; and dopamine to attention, motivation, pleasure, and reward, as well as interest in life." The proponents of this hypothesis recommend choosing the antidepressant with the mechanism of action impacting the most prominent symptoms. Anxious or irritable patients should be treated with SSRIs or norepinephrine reuptake inhibitors, and the ones with the loss of energy and enjoyment of life—with norepinephrine and dopamine enhancing drugs.[21]

Consistent with the monoamine hypothesis, a longitudinal study uncovered a moderating effect of the serotonin transporter (5-HTT) gene on stressful life events in predicting depression. Specifically, depression seems especially likely to follow stressful life events, but even more so for people with one or two short alleles of the 5-HTT gene.[2] Serotonin may help to regulate other neurotransmitter systems, and decreased serotonin activity may "permit" these systems to act in unusual and erratic ways. Facets of depression may be emergent properties of this dysregulation.[23]
Various abnormalities have been observed in dopaminergic systems however results have been inconsistent. Patients with MDD have an increased reward response to D-amphetamine compared to controls, and it has been suggested that this results from hypersensitivity of dopaminergic pathways due to natural hypoactivity. Polymorphisms of the D4 and D3 receptor have been implicated in depression further suggesting a role of dopamine in MDD. Results from CSF HVA and postmortem studies have not been consistent, but various dopamine receptor agonist show promise in treating MDD[24] There is some evidence that there is decreased nigrostriatal activity in those with melancholic depression(psychomotor retardation).[25]
Monoamine oxidase
An offshoot of the monoamine hypothesis suggests that monoamine oxidase A (MAO-A), an enzyme which metabolizes monoamines, may be overly active in depressed people. This would, in turn, cause the lowered levels of monoamines. This hypothesis received support from a PET study, which found significantly elevated activity of MAO-A in the brain of some depressed people.[26] In genetic studies, the alterations of MAO-A-related genes have not been consistently associated with depression.[27][28] Contrary to the assumptions of the monoamine hypothesis, lowered but not heightened activity of MAO-A was associated with the depressive symptoms in youth. This association was observed only in maltreated youth, indicating that both biological (MAO genes) and psychological (maltreatment) factors are important in the development of depressive disorders.[29] In addition, some evidence indicates that problems in information processing within neural networks, rather than changes in chemical balance, might underlie depression.[30]
Limitations
Since the 1990s, research has uncovered multiple limitations of the monoamine hypothesis, and its inadequacy has been criticized within the psychiatric community.[31] For one thing, serotonin system dysfunction cannot be the sole cause of depression; antidepressants usually increase synaptic serotonin very quickly, but it often takes at least two to four weeks before mood improves significantly. One possible explanation for this lag is that the neurotransmitter activity enhancement is the result of auto receptor desensitization rather which can take weeks.[32] Intensive investigation has failed to find convincing evidence of a primary dysfunction of a specific monoamine system in patients with major depressive disorders. The antidepressants that do not act through the monoamine system, such as tianeptine and opipramol, have been known for a long time. There has also been inconsistency with regards to serum 5-HIAA levels, a metabolite of serotonin.[33] Experiments with pharmacological agents that cause depletion of monoamines have shown that this depletion does not cause depression in healthy people.[34][35] However depletion of tryptophan, tyrosine and phenylalanine does result in decreased mood in those with a predisposition to depression[36] Already limited, the monoamine hypothesis has been further oversimplified when presented to the general public.[37]
Receptor binding
As of 2012, efforts to determine differences in neurotransmitter receptor expression or for function in the brains of people with MDD using positron emission tomography (PET) had shown inconsistent results. Using the PET imaging technology and reagents available as of 2012, it appeared that the D1 receptor may be underexpressed in the striatum of people with MDD.[38]
Emotional Processing
Studies of emotional processing in patients with MDD show various biases such as a tendency to rate happy faces more negatively.[39] Functional neuroimaging has demonstrated hyperactivity of various brain regions in response to negative emotional stimuli, and hypoactivity in response to positive stimuli. Patients also showed decreased activity in the left dorsolateral prefrontal cortex in response to negative stimuli.[40]
Brain regions
Research on the brains of depressed patients usually shows disturbed patterns of interaction between multiple parts of the brain. Several areas of the brain are implicated in studies seeking to more fully understand the biology of depression:
Raphe nuclei
The sole source of serotonin in the brain is the raphe nuclei, a group of small nerve cell nuclei in the upper brain stem, located directly at the mid-line of the brain. There is some evidence for neuropathological abnormalities in the rostral raphe nuclei in depression.[41][42][43] Despite their small size, they reach very widely through their projections, and are involved in a very diverse set of functions. Most antidepressants are serotonergic.
Subgenual cingulate
Recent studies have shown that Brodmann area 25, also known as Subgenual cingulate is metabolically overactive in treatment-resistant depression.[44] This region is extremely rich in serotonin transporters and is considered as a governor for a vast network involving areas like hypothalamus and brain stem, which influences changes in appetite and sleep; the amygdala and insula, which affect the mood and anxiety; the hippocampus, which plays an important role in memory formation; and some parts of the frontal cortex responsible for self-esteem.[45][46] Thus disturbances in this area or a smaller than normal size of this area contributes to depression. Deep Brain Stimulations of this area have been successful in reducing its elevated activity and thus curing depression in patients that could not be cured by anti-depressants.[47] One meta analysis found this area to be overactive in youth with MDD compared to controls[48]
Hypothalamic-pituitary-adrenal axis
The hypothalamic-pituitary-adrenal axis is a chain of endocrine structures that are activated during the body's response to stressors of various sorts. It often shows increased activation in depressed people, but the mechanism behind this is not yet known.[49] Increased basal cortisol levels and abnormal response to dexamethasone challenges have been observed in patients with depression.[50] Early life stress has been hypothesized as a potential cause of HPA dysfunction.[51][52]
Ventricles
Multiple studies have found evidence of ventricular enlargement in people who have depression, particularly enlargement of the third ventricle.[53][54][55] These observations are interpreted as indicating loss of neural tissue in brain regions adjacent to the enlarged ventricle, leading to suggestions that cytokines and related mediators of neurodegeneration may play a role in giving rise to the disease.[56][57][58]
Prefrontal cortex
One review reported hypoactivity in the prefrontal cortex of those with depression compared to controls.[59] The prefrontal cortex is involved in emotional processing and regulation, and dysfunction of this process may be involved in the etiology of depression. One study on antidepressant treatment found an increase in PFC activity in response to administration of antidepressants[60] One meta analysis published in 2012 found that areas of the prefrontal cortex were underachieve in response to negative stimuli in depressed patients.[61]
Amygdala
The amygdala, a structure involved in emotional processing appears to be hyperactive in those with major depressive disorder.[48]
Altered neuroplasticity
Recent studies have called attention to the role of altered neuroplasticity in depression. A review found convergence of three phenomena:
- Chronic stress reduces synaptic and dendritic plasticity
- Depressed subjects show evidence of impaired neuroplasticity (e.g. shortening and reduced complexity of dendritic trees)
- Anti-depressant medications enhance neuroplasticity at both a molecular and dendritic level.
The conclusion is that disrupted neuroplasticity is an underlying feature of depression, and is reversed by antidepressants.[62]
Inflammation
Various review have found that general inflammation may play a role in depression.[63][64] One meta analysis of cytokines in depressed patients found increased IL-6 and TNF-a levels relative to controls.[65] First theories came about when it was noticed that interferon therapy caused depression in a large number of patients.[66] Cytokines by manipulating neurotransmitters are involved in the generation of sickness behavior, which shares some overlap with the symptoms of depression. Neurotransmitters hypothesized to be affected include dopamine and serotonin, which are common targets for antidepressant drugs. One review found normalization of cytokine levels after successful treatment of depression.[67] Various sources of inflammation in depressive illness have been hypothesized and include trauma, sleep problems, diet, smoking and obesity.[68]
A meta analysis published in 2014 found the use of anti-inflammatory drugs such as NSAIDs and investigational cytokine inhibitors reduced depressive symptoms[69]
Large-scale brain network theory
Instead of studying one brain region, studying large scale brain networks is another approach to understanding psychiatric and neurological disorders,[70] supported by recent research that has shown that multiple brain regions are involved in these disorders. Understanding the disruptions in these networks may provide important insights into interventions for treating these disorders. Recent work suggests that at least three large-scale brain networks are important in psychopathology:[70]
Central executive network
The executive network is made up of fronto-parietal regions, including dorsolateral prefrontal cortex and lateral posterior parietal cortex.[71][72] This network is crucially involved in high level cognitive functions such as maintaining and using information in working memory, problem solving, and decision making.[70][73][74][75][76] Deficiencies in this network are common in most major psychiatric and neurological disorders, including depression.[77][78] Because this network is crucial for everyday life activities, those who are depressed can show impairment in basic activities like test taking and being decisive.[79]
Default mode network
The default mode network includes hubs in the prefrontal cortex and posterior cingulate, with other prominent regions of the network in the medial temporal lobe and angular gyrus.[70] The default mode network is usually active during mind-wandering and thinking about social situations. In contrast, during specific tasks probed in cognitive science (for example, simple attention tasks), the default network is often deactivated.[80][81] Research has shown that regions in the default mode network (including medial prefrontal cortex and posterior cingulate) show greater activity when depressed participants ruminate (that is, when they engage in repetitive self-focused thinking) than when typical, healthy participants ruminate.[82] Individuals suffering from major depression also show increased connectivity between the default mode network and the subgenual cingulate and the adjoining ventromedial prefrontal cortex in comparison to healthy individuals, individuals with dementia or with autism. Numerous studies suggest that the subgenual cingulate plays an important role in the dysfunction that characterizes major depression.[83] The increased activation in the default mode network during rumination and the atypical connectivity between core default mode regions and the subgenual cingulate may underlie the tendency for depressed individual to get “stuck” in the negative, self-focused thoughts that often characterize depression.[84] However, further research is needed to gain a precise understanding of how these network interactions map to specific symptoms of depression.
Salience network
The salience network is a cingulate-frontal operculum network that includes core nodes in the anterior cingulate and anterior insula.[71] A salience network is a large-scale brain network involved in detecting and orienting the most pertinent of the external stimuli and internal events being presented.[70] Individuals who have a tendency to experience negative emotional states (scoring high on measures of neuroticism) show an increase in the right anterior insula during decision-making, even if the decision has already been made.[85] This atypically high activity in the right anterior insula is thought to contribute to the experience of negative and worrisome feelings.[86] In major depressive disorder, anxiety is often a part of the emotional state that characterizes depression.[87]
References
- ↑ Nierenberg, AA (2009). "The long tale of the short arm of the promoter region for the gene that encodes the serotonin uptake protein" (PDF). CNS spectrums. 14 (9): 462–3. PMID 19890228.
- 1 2 3 Caspi, Avshalom; Sugden, Karen; Moffitt, Terrie E.; Taylor, Alan; Craig, Ian W.; Harrington, HonaLee; McClay, Joseph; Mill, Jonathan; Martin, Judy; Braithwaite, Antony; Poulton, Richie (July 2003). "Influence of Life Stress on Depression: Moderation by a Polymorphism in the 5-HTT Gene". Science. 301 (5631): 386–89. Bibcode:2003Sci...301..386C. doi:10.1126/science.1083968. PMID 12869766.
- ↑ Kendler, K.; Kuhn, J.; Vittum, J.; Prescott, C.; Riley, B. (2005). "The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression: a replication". Archives of General Psychiatry. 62 (5): 529–535. doi:10.1001/archpsyc.62.5.529. PMID 15867106. Lay summary – New Hot Paper Comments (6 September 2006).
- ↑ Gillespie, N. A.; Whitfield, J. B.; Williams, B.; Heath, A. C.; Martin, N. G. (2005). "The relationship between stressful life events, the serotonin transporter (5-HTTLPR) genotype and major depression". Psychological Medicine. 35 (1): 101–111. doi:10.1017/S0033291704002727. PMID 15842033.
- ↑ Surtees, P.; Wainwright, N.; Willis-Owen, S.; Luben, R.; Day, N.; Flint, J. (2006). "Social adversity, the serotonin transporter (5-HTTLPR) polymorphism and major depressive disorder". Biological Psychiatry. 59 (3): 224–229. doi:10.1016/j.biopsych.2005.07.014. PMID 16154545.
- ↑ Uher, R.; McGuffin, P. (2008). "The moderation by the serotonin transporter gene of environmental adversity in the aetiology of mental illness: review and methodological analysis". Molecular Psychiatry. 13 (2): 131–146. doi:10.1038/sj.mp.4002067. PMID 17700575.
- ↑ Risch, N.; Herrell, R.; Lehner, T.; Liang, K.; Eaves, L.; Hoh, J.; Griem, A.; Kovacs, M.; Ott, J.; Merikangas, K. R. (2009). "Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: a meta-analysis". Journal of the American Medical Association. 301 (23): 2462–2471. doi:10.1001/jama.2009.878. PMC 2938776
 . PMID 19531786.
. PMID 19531786. - ↑ Munafo, M.; Durrant, C.; Lewis, G.; Flint, J. (2009). "Gene × Environment Interactions at the Serotonin Transporter Locus". Biological Psychiatry. 65 (3): 211–219. doi:10.1016/j.biopsych.2008.06.009. PMID 18691701.
- ↑ Uher, R.; McGuffin, P. (2010). "The moderation by the serotonin transporter gene of environmental adversity in the etiology of depression: 2009 update". Molecular Psychiatry. 15 (1): 18–22. doi:10.1038/mp.2009.123. PMID 20029411.
- ↑ Levinson, D. (2006). "The genetics of depression: a review". Biological Psychiatry. 60 (2): 84–92. doi:10.1016/j.biopsych.2005.08.024. PMID 16300747.
- ↑ Dwivedi Y (2009). "Brain-derived neurotrophic factor: role in depression and suicide". Neuropsychiatr Dis Treat. 5: 433–49. doi:10.2147/NDT.S5700. PMC 2732010
 . PMID 19721723.
. PMID 19721723. - 1 2 Krishnan, V.; Nestler, E. (2008). "The molecular neurobiology of depression". Nature. 455 (7215): 894–902. Bibcode:2008Natur.455..894K. doi:10.1038/nature07455. PMC 2721780
 . PMID 18923511.
. PMID 18923511. - ↑ Pezawas, L.; Meyer-Lindenberg, A.; Goldman, A. L.; Verchinski, B. A.; Chen, G.; Kolachana, B. S.; Egan, M. F.; Mattay, V. S.; Hariri, A. R.; Weinberger, D. R. (2008). "Evidence of biologic epistasis between BDNF and SLC6A4 and implications for depression". Molecular Psychiatry. 13 (7): 709–716. doi:10.1038/mp.2008.32. PMID 18347599.
- ↑ Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium; Ripke, S; Wray, N. R.; Lewis, C. M.; Hamilton, S. P.; Weissman, M. M.; Breen, G; Byrne, E. M.; Blackwood, D. H.; Boomsma, D. I.; Cichon, S; Heath, A. C.; Holsboer, F; Lucae, S; Madden, P. A.; Martin, N. G.; McGuffin, P; Muglia, P; Noethen, M. M.; Penninx, B. P.; Pergadia, M. L.; Potash, J. B.; Rietschel, M; Lin, D; Müller-Myhsok, B; Shi, J; Steinberg, S; Grabe, H. J.; Lichtenstein, P; et al. (2013). "A mega-analysis of genome-wide association studies for major depressive disorder". Molecular Psychiatry. 18 (4): 497–511. doi:10.1038/mp.2012.21. PMC 3837431
 . PMID 22472876.
. PMID 22472876. - ↑ Converge Consortium; Bigdeli, Tim B.; Kretzschmar, Warren; Li, Yihan; Liang, Jieqin; Song, Li; Hu, Jingchu; Li, Qibin; Jin, Wei; Hu, Zhenfei; Wang, Guangbiao; Wang, Linmao; Qian, Puyi; Liu, Yuan; Jiang, Tao; Lu, Yao; Zhang, Xiuqing; Yin, Ye; Li, Yingrui; Xu, Xun; Gao, Jingfang; Reimers, Mark; Webb, Todd; Riley, Brien; Bacanu, Silviu; Peterson, Roseann E.; Chen, Yiping; Zhong, Hui; Liu, Zhengrong; et al. (2015). "Sparse whole-genome sequencing identifies two loci for major depressive disorder". Nature. 523 (7562): 588–91. doi:10.1038/nature14659. PMC 4522619
 . PMID 26176920.
. PMID 26176920. - 1 2 Carlson, Neil R. (2013). Physiology of behavior (11th ed.). Boston: Pearson. pp. 578–582. ISBN 978-0-205-23939-9. OCLC 769818904.
- 1 2 3 Adrien J.. Neurobiological bases for the relation between sleep and depression. Sleep Medicine Review. 2003;6(5):341–51. doi:10.1053/smrv.2001.0200. PMID 12531125.
- 1 2 Terman M. Evolving applications of light therapy. Sleep Medicine Review. 2007;11(6):497–507. doi:10.1016/j.smrv.2007.06.003. PMID 17964200.
- ↑ Benedetti F, Barbini B, Colombo C, Smeraldi E. Chronotherapeutics in a psychiatric ward. Sleep Medicine Review. 2007;11(6):509–22. doi:10.1016/j.smrv.2007.06.004. PMID 17689120.
- ↑ Carlson, Neil R. (2005). Foundations of Physiological Psychology (6th ed.). Boston: Pearson A and B. p. 108. ISBN 0-205-42723-5. OCLC 60880502.
- ↑ Nutt DJ (2008). "Relationship of neurotransmitters to the symptoms of major depressive disorder". Journal of Clinical Psychiatry. 69 Suppl E1: 4–7. PMID 18494537.
- ↑ Carlson, N. (2013). Physiology of behavior. (11 ed., pp. 575-576). United States of America: Pearson.
- ↑ Mandell AJ, Knapp S (1979). "Asymmetry and mood, emergent properties of serotonin regulation: A proposed mechanism of action of lithium". Archives of General Psychiatry. 36 (8): 909–16. doi:10.1001/archpsyc.1979.01780080083019. PMID 454111.
- ↑ Dunlop, Boadie W.; Nemeroff, Charles B. (1 April 2007). "The Role of Dopamine in the Pathophysiology of Depression". Archives of General Psychiatry. 64 (3). doi:10.1001/archpsyc.64.3.327. ISSN 0003-990X.
- ↑ Willner, Paul (1 December 1983). "Dopamine and depression: A review of recent evidence. I. Empirical studies". Brain Research Reviews. 6 (3): 211–224. doi:10.1016/0165-0173(83)90005-X.
- ↑ Meyer JH, Ginovart N, Boovariwala A, et al. (November 2006). "Elevated monoamine oxidase a levels in the brain: An explanation for the monoamine imbalance of major depression". Archives of General Psychiatry. 63 (11): 1209–16. doi:10.1001/archpsyc.63.11.1209. PMID 17088501.
- ↑ Huang SY, Lin MT, Lin WW, Huang CC, Shy MJ, Lu RB (2007-12-19). "Association of monoamine oxidase A (MAOA) polymorphisms and clinical subgroups of major depressive disorders in the Han Chinese population". World Journal of Biological Psychiatry. Informa Healthcare. 10 (4 Pt 2): 544–51. doi:10.1080/15622970701816506. PMID 19224413. Retrieved 2008-09-20.
- ↑ Yu YW, Tsai SJ, Hong CJ, Chen TJ, Chen MC, Yang CW (September 2005). "Association study of a monoamine oxidase a gene promoter polymorphism with major depressive disorder and antidepressant response". Neuropsychopharmacology. 30 (9): 1719–23. doi:10.1038/sj.npp.1300785. PMID 15956990.
- ↑ Cicchetti D, Rogosch FA, Sturge-Apple ML (2007). "Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptomatology among adolescents from low socioeconomic status backgrounds". Dev. Psychopathol. 19 (4): 1161–80. doi:10.1017/S0954579407000600. PMID 17931441.
- ↑ Castrén, E (2005). "Is mood chemistry?". Nature Reviews Neuroscience. 6 (3): 241–46. doi:10.1038/nrn1629. PMID 15738959.
- ↑ Hirschfeld RM (2000). "History and evolution of the monoamine hypothesis of depression". Journal of Clinical Psychiatry. 61 Suppl 6: 4–6. PMID 10775017.
- ↑ al.], editors, Kenneth L. Davis ... [et (2002). Neuropsychopharmacology : the fifth generation of progress : an official publication of the American College of Neuropsychopharmacology (5th ed. ed.). Philadelphia, Pa.: Lippincott Williams & Wilkins. pp. 1139–1163. ISBN 9780781728379.
- ↑ Jacobsen, Jacob P. R.; Medvedev, Ivan O.; Caron, Marc G. (5 September 2012). "The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse". Philosophical Transactions of the Royal Society B: Biological Sciences. 367 (1601): 2444–2459. doi:10.1098/rstb.2012.0109. ISSN 0962-8436.
- ↑ Delgado PL, Moreno FA (2000). "Role of norepinephrine in depression". J Clin Psychiatry. 61 Suppl 1: 5–12. PMID 10703757.
- ↑ Delgado PL (2000). "Depression: the case for a monoamine deficiency". Journal of Clinical Psychiatry. 61 Suppl 6: 7–11. PMID 10775018.
- ↑ Ruhe, HG; Mason, NS; Schene, AH (2007). "Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies". Molecular Psychiatry. 12: 331–359. doi:10.1038/sj.mp.4001949. PMID 17389902.
- ↑ Lacasse, Jeffrey R.; Leo, Jonathan (8 November 2005). "Serotonin and Depression: A Disconnect between the Advertisements and the Scientific Literature". PLoS Medicine. 2 (12): e392. doi:10.1371/journal.pmed.0020392. PMC 1277931
 . PMID 16268734.
. PMID 16268734. 
- ↑ Savitz, Jonathan; Drevets, Wayne (2013). "Neuroreceptor imaging in depression". Neurobiology of Disease. 52: 49–65. doi:10.1016/j.nbd.2012.06.001. PMID 22691454.
- ↑ Bourke, Cecilia; Douglas, Katie; Porter, Richard (1 August 2010). "Processing of facial emotion expression in major depression: a review". The Australian and New Zealand Journal of Psychiatry. 44 (8): 681–696. doi:10.3109/00048674.2010.496359. ISSN 1440-1614.
- ↑ Groenewold, Nynke A.; Opmeer, Esther M.; de Jonge, Peter; Aleman, André; Costafreda, Sergi G. (1 February 2013). "Emotional valence modulates brain functional abnormalities in depression: evidence from a meta-analysis of fMRI studies". Neuroscience and Biobehavioral Reviews. 37 (2): 152–163. doi:10.1016/j.neubiorev.2012.11.015. ISSN 1873-7528.
- ↑ Underwood, MD; Khaibulina, AA; Ellis, SP; Moran, A; Rice, PM; Mann, JJ; Arango, V (15 August 1999). "Morphometry of the dorsal raphe nucleus serotonergic neurons in suicide victims". Biol Psychiatry. 46 (4): 473–83. doi:10.1016/S0006-3223(99)00043-8. PMID 10459396.

- ↑ Arango, V; Underwood, MD; Boldrini, M; Tamir, H; Kassir, SA; Hsiung, S; Chen, JJ; Mann, JJ (December 2001). "Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims". Neuropsychopharmacology. 25 (6): 892–903. doi:10.1016/S0893-133X(01)00310-4. PMID 11750182.
- ↑ Matthews, PR; Harrison, PJ (March 2012). "A morphometric, immunohistochemical, and in situ hybridization study of the dorsal raphe nucleus in major depression, bipolar disorder, schizophrenia, and suicide". J Affect Disord. 137 (1–3): 125–134. doi:10.1016/j.jad.2011.10.043. PMC 3314923
 . PMID 22129767.
. PMID 22129767. 
- ↑ Mayberg, HS; Brannan, SK; Tekell, JL; Silva, JA; Mahurin, RK; McGinnis, S; Jerabek, PA (15 October 2000). "Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response". Biol Psychiatry. 48 (8): 830–43. doi:10.1016/s0006-3223(00)01036-2. PMID 11063978. (subscription required)
- ↑ Carlson, N. (2013). Physiology of behavior. (11 ed., pp. 576-578). United States of America: Pearson.
- ↑ Insel, Thomas R. (April 2010). "Faulty Circuits". Scientific American. 302 (4): 44–51. doi:10.1038/scientificamerican0410-44. PMID 20349573. (subscription required)
- ↑ Mayberg, Helen S.; Lozano, Andres M.; Voon, Valerie; McNeely, Heather E.; Seminowicz, David; Hamani, Clement; Schwalb, Jason M.; Kennedy, Sidney H. (3 March 2005). "Deep Brain Stimulation for Treatment-Resistant Depression". Neuron. 45 (5): 651–660. doi:10.1016/j.neuron.2005.02.014. PMID 15748841.

- 1 2 Miller, Chris H.; Hamilton, J. Paul; Sacchet, Matthew D.; Gotlib, Ian H. (1 October 2015). "Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth". JAMA psychiatry. 72 (10): 1045–1053. doi:10.1001/jamapsychiatry.2015.1376. ISSN 2168-6238.
- ↑ Pariante CM, Lightman SL (September 2008). "The HPA axis in major depression: classical theories and new developments.". Trends Neurosci. 31 (9): :464–468. doi:10.1016/j.tins.2008.06.006. PMID 18675469.
- ↑ Belvederi Murri, Martino; Pariante, Carmine; Mondelli, Valeria; Masotti, Mattia; Atti, Anna Rita; Mellacqua, Zefiro; Antonioli, Marco; Ghio, Lucio; Menchetti, Marco; Zanetidou, Stamatula; Innamorati, Marco; Amore, Mario (1 March 2014). "HPA axis and aging in depression: systematic review and meta-analysis". Psychoneuroendocrinology. 41: 46–62. doi:10.1016/j.psyneuen.2013.12.004. ISSN 1873-3360.
- ↑ Juruena, Mario F. (1 September 2014). "Early-life stress and HPA axis trigger recurrent adulthood depression". Epilepsy & Behavior: E&B. 38: 148–159. doi:10.1016/j.yebeh.2013.10.020. ISSN 1525-5069.
- ↑ Heim, Christine; Newport, D. Jeffrey; Mletzko, Tanja; Miller, Andrew H.; Nemeroff, Charles B. (1 August 2008). "The link between childhood trauma and depression: Insights from HPA axis studies in humans". Psychoneuroendocrinology. 33 (6): 693–710. doi:10.1016/j.psyneuen.2008.03.008. ISSN 0306-4530.
- ↑ Hendrie, C.A.; Pickles, A.R. (2009). "Depression as an evolutionary adaptation: Implications for the development of preclinical models". Medical Hypotheses. 72 (3): 342–347. doi:10.1016/j.mehy.2008.09.053. PMID 19153014. Retrieved September 25, 2013.
- ↑ Hendrie, C.A.; Pickles, A.R. (2010). "Depression as an evolutionary adaptation: Anatomical organisation around the third ventricle". Medical Hypotheses. 74 (4): 735–740. doi:10.1016/j.mehy.2009.10.026. PMID 19931308. Retrieved September 25, 2013.
- ↑ Sheline, Yvette (August 2003). "Neuroimaging studies of mood disorder effects on the brain". Biological Psychiatry. 54 (3): 338–352. doi:10.1016/s0006-3223(03)00347-0. PMID 12893109. Retrieved September 25, 2013.
- ↑ Manji, Husseini K.; Quiroz, Jorge A.; Sporn, Jonathan; Payne, Jennifer L.; Denicoff, Kirk; Gray, Neil A.; Zarate Jr., Carlos A.; Charney, Dennis S. (April 2003). "Enhancing neuronal plasticity and cellular resilience to develop novel, improved therapeutics for difficult-to-treat depression". Biological Psychiatry. 53: 707–742. doi:10.1016/s0006-3223(03)00117-3. Retrieved September 25, 2013.
- ↑ Miller, A. H.; Maletic, V.; Raison, C. L. (2009). "Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression". Biological Psychiatry. 65 (9): 732–741. doi:10.1016/j.biopsych.2008.11.029. PMID 19150053.
- ↑ Raison, C. L.; Capuron, L.; Miller, A. H. (2006). "Cytokines sing the blues: inflammation and the pathogenesis of depression". Trends in Immunology. 27 (1): 24–31. doi:10.1016/j.it.2005.11.006. PMC 3392963
 . PMID 16316783.
. PMID 16316783. - ↑ Wessa, Michèle; Lois, Giannis (30 November 2016). "Brain Functional Effects of Psychopharmacological Treatment in Major Depression: A Focus on Neural Circuitry of Affective Processing". Current Neuropharmacology. 13 (4): 466–479. doi:10.2174/1570159X13666150416224801. ISSN 1570-159X.
- ↑ Outhred, Tim; Hawkshead, Brittany E.; Wager, Tor D.; Das, Pritha; Malhi, Gin S.; Kemp, Andrew H. (1 September 2013). "Acute neural effects of selective serotonin reuptake inhibitors versus noradrenaline reuptake inhibitors on emotion processing: Implications for differential treatment efficacy". Neuroscience and Biobehavioral Reviews. 37 (8): 1786–1800. doi:10.1016/j.neubiorev.2013.07.010. ISSN 1873-7528.
- ↑ Hamilton, J. Paul; Etkin, Amit; Furman, Daniella J.; Lemus, Maria G.; Johnson, Rebecca F.; Gotlib, Ian H. "Functional Neuroimaging of Major Depressive Disorder: A Meta-Analysis and New Integration of Baseline Activation and Neural Response Data". American Journal of Psychiatry. 169 (7): 693–703. doi:10.1176/appi.ajp.2012.11071105.
- ↑ Christopher Pittenger; Ronald S Duman (2008). "Stress, Depression, and Neuroplasticity: A Convergence of Mechanisms". Neuropsychopharmacology. 33 (1): 88–109. doi:10.1038/sj.npp.1301574. PMID 17851537
- ↑ Krishnadas, Rajeev; Cavanagh, Jonathan (1 May 2012). "Depression: an inflammatory illness?". Journal of Neurology, Neurosurgery, and Psychiatry. 83 (5): 495–502. doi:10.1136/jnnp-2011-301779. ISSN 1468-330X.
- ↑ Patel, Amisha (1 September 2013). "Review: the role of inflammation in depression". Psychiatria Danubina. 25 Suppl 2: S216–223. ISSN 0353-5053.
- ↑ Dowlati, Yekta; Herrmann, Nathan; Swardfager, Walter; Liu, Helena; Sham, Lauren; Reim, Elyse K.; Lanctôt, Krista L. (1 March 2010). "A meta-analysis of cytokines in major depression". Biological Psychiatry. 67 (5): 446–457. doi:10.1016/j.biopsych.2009.09.033. ISSN 1873-2402.
- ↑ Dantzer, Robert; O’Connor, Jason C.; Freund, Gregory G.; Johnson, Rodney W.; Kelley, Keith W. (3 December 2016). "From inflammation to sickness and depression: when the immune system subjugates the brain". Nature reviews. Neuroscience. 9 (1): 46–56. doi:10.1038/nrn2297. ISSN 1471-003X.
- ↑ Raedler, Thomas J. (1 November 2011). "Inflammatory mechanisms in major depressive disorder". Current Opinion in Psychiatry. 24 (6): 519–525. doi:10.1097/YCO.0b013e32834b9db6. ISSN 1473-6578.
- ↑ Berk, Michael; Williams, Lana J; Jacka, Felice N; O’Neil, Adrienne; Pasco, Julie A; Moylan, Steven; Allen, Nicholas B; Stuart, Amanda L; Hayley, Amie C; Byrne, Michelle L; Maes, Michael (12 September 2013). "So depression is an inflammatory disease, but where does the inflammation come from?". BMC Medicine. 11: 200. doi:10.1186/1741-7015-11-200. ISSN 1741-7015.
- ↑ Köhler, Ole; Benros, Michael E.; Nordentoft, Merete; Farkouh, Michael E.; Iyengar, Rupa L.; Mors, Ole; Krogh, Jesper (1 December 2014). "Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials". JAMA psychiatry. 71 (12): 1381–1391. doi:10.1001/jamapsychiatry.2014.1611. ISSN 2168-6238.
- 1 2 3 4 5 Menon, Vinod (October 2011). "Large-scale brain networks and psychopathology: a unifying triple network model". Trends in Cognitive Sciences. 15 (10): 483–506. doi:10.1016/j.tics.2011.08.003. PMID 21908230.
- 1 2 Seeley, W.W; et al. (February 2007). "Dissociable intrinsic connectivity networks for salience processing and executive control". The Journal of Neuroscience. 27.
- ↑ Habas, C; et al. (1 July 2009). "Distinct cerebellar contributions to intrinsic connectivity networks". The Journal of Neuroscience. 29.
- ↑ Petrides, M (2005). "Lateral prefrontal cortex: architecture and functional organization". Philosophical Transactions of the Royal Society B. 360 (1456): 781–795. doi:10.1098/rstb.2005.1631.
- ↑ Koechlin, E; Summerfield, C (2007). "An information theoretical approach to prefrontal executive function". Trends in Cognitive Sciences. 11 (6): 229–235. doi:10.1016/j.tics.2007.04.005. PMID 17475536.
- ↑ Miller, E.K.; Cohen, J.D. (2001). "An integrative theory of prefrontal cortex function". Annual Review of Neuroscience. 24: 167–202. doi:10.1146/annurev.neuro.24.1.167. PMID 11283309.
- ↑ Muller, N.G.; Knight, R.T. (2006). "The functional neuroanatomy of working memory: contributions of human brain lesion studies". Neuroscience. 139 (1): 51–58. doi:10.1016/j.neuroscience.2005.09.018. PMID 16352402.
- ↑ Woodward, N.D.; et al. (2011). "Functional resting-state networks are differentially affected in schizophrenia". Schizophrenia Research. 130 (1–3): 86–93. doi:10.1016/j.schres.2011.03.010. PMC 3139756
 . PMID 21458238.
. PMID 21458238. - ↑ Menon, Vinod; et al. (2001). "Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms". NeuroImage. 13 (3): 433–446. doi:10.1006/nimg.2000.0699. PMID 11170809.
- ↑ Levin, R.L.; et al. (2007). "Cognitive deficits in depression and functional specificity of regional brain activity". Cognitive Therapy and Research. 31 (2): 211–233. doi:10.1007/s10608-007-9128-z.
- ↑ Qin, P; Northoff, G (2011). "How is our self related to midline regions and the default mode network?". NeuroImage. 57 (3): 1221–1233. doi:10.1016/j.neuroimage.2011.05.028. PMID 21609772.
- ↑ Raichle, M.E.; et al. (2001). "A default mode of brain function". Proceedings of the National Academy of Sciences of the United States of America. 98 (2): 676–682. doi:10.1073/pnas.98.2.676. PMC 14647
 . PMID 11209064.
. PMID 11209064. - ↑ Cooney, R.E.; et al. (2010). "Neural correlates of rumination in depression". Cognitive Affective and Behavioral Neuroscience. 10 (4): 470–478. doi:10.3758/cabn.10.4.470.
- ↑ Broyd, S.J.; et al. (2009). "Default mode brain dysfunction in mental disorders: a systematic review". Neuroscience & Biobehavioral Reviews. 33 (3): 279–296. doi:10.1016/j.neubiorev.2008.09.002. PMID 18824195.
- ↑ Hamani, C; et al. (15 February 2011). "The subcallosal cingulate gyrus in the context of major depression". Biological Psychiatry. 69 (4): 301–8. doi:10.1016/j.biopsych.2010.09.034. PMID 21145043.
- ↑ Feinstein, J.S.; et al. (September 2006). "Anterior insula reactivity during certain decisions is associated with neuroticism". Social Cognition and Affective Neuroscience. 1 (2): 136–142. doi:10.1093/scan/nsl016.
- ↑ Paulus, M.P; Stein, M.B. (2006). "An insular view of anxiety". Biological Psychiatry. 60 (4): 383–387. doi:10.1016/j.biopsych.2006.03.042. PMID 16780813.
- ↑ Antony, M.M. (2009). Oxford Handbook of Anxiety and Related Disorders. Oxford University Press.
Further reading
- Szafran, K; Faron-Górecka, A; Kolasa, M; Kuśmider, M; Solich, J; Zurawek, D; Dziedzicka-Wasylewska, M (2013). "Potential role of G protein-coupled receptor (GPCR) heterodimerization in neuropsychiatric disorders: a focus on depression" (PDF). Pharmacol Rep. 65 (6): 1498–505. doi:10.1016/s1734-1140(13)71510-x. PMID 24552997.
- Naumenko, VS; Popova, NK; Lacivita, E; Leopoldo, M; Ponimaskin, EG (July 2014). "Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders". CNS Neurosci Ther. 20 (7): 582–90. doi:10.1111/cns.12247. PMID 24935787.