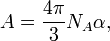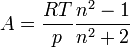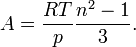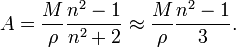Molar refractivity
Molar refractivity,  , is a measure of the total polarizability of a mole of a substance and is dependent on the temperature, the index of refraction, and the pressure.
, is a measure of the total polarizability of a mole of a substance and is dependent on the temperature, the index of refraction, and the pressure.
The molar refractivity is defined as
where 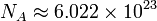 is the Avogadro constant and
is the Avogadro constant and 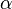 is the mean polarizability of a molecule.
is the mean polarizability of a molecule.
Substituting the molar refractivity into the Lorentz-Lorenz formula gives
For a gas, 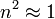 , so the molar refractivity can be approximated by
, so the molar refractivity can be approximated by
In SI units, 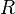 has units of J mol−1 K−1,
has units of J mol−1 K−1, 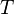 has units K,
has units K,  has no units, and
has no units, and 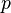 has units of Pa, so the units of
has units of Pa, so the units of  are m3 mol−1.
are m3 mol−1.
In terms of density, ρ molecular weight, M it can be shown that:
References
- Born, Max, and Wolf, Emil, Principles of Optics: Electromagnetic Theory of Propagation, Interference and Diffraction of Light (7th ed.), section 2.3.3, Cambridge University Press (1999) ISBN 0-521-64222-1
This article is issued from Wikipedia - version of the 12/2/2013. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.