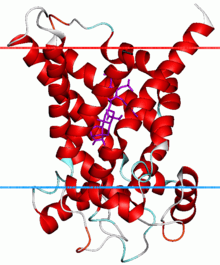
Mitochondrial carrier
|
Mitochondrial ADP/ATP carrier | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Mito_carr | ||||||||
| Pfam | PF00153 | ||||||||
| InterPro | IPR001993 | ||||||||
| PROSITE | PDOC00189 | ||||||||
| SCOP | 1okc | ||||||||
| SUPERFAMILY | 1okc | ||||||||
| TCDB | 2.A.29 | ||||||||
| OPM superfamily | 21 | ||||||||
| OPM protein | 1okc | ||||||||
| |||||||||
Mitochondrial carriers are proteins from a solute carrier family which transfer molecules across the membranes of the mitochondria.[1] Mitochondrial carriers are also classified in the Transporter Classification Database. The Mitochondrial Carrier (MC) Superfamily has been expanded to include both the original Mitochondrial Carrier (MC) family (TC# 2.A.29) and the Mitochondrial Inner/Outer Membrane Fusion (MMF) family (TC# 9.B.25).[2]
Phylogeny
Members of the MC family (TC# 2.A.29) are found exclusively in eukaryotic organelles although they are nuclearly encoded. Most are found in mitochondria, but some are found in peroxisomes of animals, in hydrogenosomes of anaerobic fungi, and in amyloplasts of plants.
15 paralogues of the MC family are encoded within the genome of Saccharomyces cerevisiae. 50 have been identified in humans, 58 in A. thaliana and 35 in S. cerevisiae. The functions of many of the human homologues are unknown, but most of the yeast homologues have been functionally identified.[3][4] See TCDB for functional assignments
Function
Many MC proteins preferentially catalyze the exchange of one solute for another (antiport). A variety of these substrate carrier proteins, which are involved in energy transfer, have been found in the inner membranes of mitochondria and other eukaryotic organelles such as the peroxisome and facilitate the transport of inorganic ions, nucleotides, amino acids, keto acids and cofactors across the membrane.[5][6][7][8] Such proteins include:
- ADP/ATP carrier protein (ADP-ATP translocase; i.e., TC# 2.A.29.1.2)
- 2-oxoglutarate/malate carrier protein (SLC25A11; TC# 2.A.29.2.11) [9]
- phosphate carrier protein (SLC25A3; TC# 2.A.29.4.2)
- Tricarboxylate transport protein (SLC25A1, or citrate transport protein; TC# 2.A.29.7.2)[9]
- Graves disease carrier protein (SLC25A16; TC# 2.A.29.12.1)
- Yeast mitochondrial proteins MRS3 (TC# 2.A.29.5.1) and MRS4 (TC# 2.A.29.5.2)
- Yeast mitochondrial FAD carrier protein (TC# 2.A.29.10.1)
- As well as many others.[10][11]
Functional aspects of these proteins, including metabolite transport, have been reviewed by Dr. Ferdinando Palmieri and Dr. Ciro Leonardo Pierri (2010).[12][13][14] Diseases caused by defects of mitochondrial carriers are reviewed by Palmieri et al. (2008) and by Gutiérrez-Aguilar and Baines 2013.[15][16] Mutations of mitochondrial carrier genes involved in mitochondrial functions other than oxidative phosphorylation are responsible for carnitine/acylcarnitine carrier deficiency, HHH syndrome, aspartate/glutamate isoform 2 deficiency, Amish microcephaly, and neonatal myoclonic epilepsy. These disorders are characterized by specific metabolic dysfunctions, depending on the physiological role of the affected carrier in intermediary metabolism. Defects of mitochondrial carriers that supply mitochondria with the substrates of oxidative phosphorylation, inorganic phosphate and ADP, are responsible for diseases characterized by defective energy production.[15] Residues involved in substrate binding in the middle of the transporter and gating have been identified and analyzed.[8]
Structure
Permeases of the MC family (the human SLC25 family) possess six transmembrane β-helical spanners. The proteins are of fairly uniform size of about 300 residues. They arose by tandem intragenic triplication in which a genetic element encoding two spanners gave rise to one encoding six spanners.[17] This event may have occurred less than 2 billion years ago when mitochondria first developed their specialized endosymbiotic functions within eukaryotic cells.[18] Members of the MC family are functional and structural monomers although early reports indicated that they are dimers.[3][4]
Most MC proteins contain a primary structure exhibiting three repeats, each of about 100 amino acyl residues in length, and both the N and C termini face the intermembrane space. All carriers contain a common sequence, referred to as the MCF motif, in each repeated region, with some variation in one or two signature sequences.[1]
Amongst the members of the mitochondrial carrier family that have been identified, it is the ADP/ATP carrier (AAC; TC# 2.A.29.1.1) that is responsible for importing ADP into the mitochondria and exporting ATP out of the mitochondria and into the cytosol following synthesis.[19] The AAC is an integral membrane protein that is synthesised lacking a cleavable presequence, but instead contains internal targeting information.[20] It forms a dimer of two identical subunits[21] and consists of a basket shaped structure with six transmembrane helices that are tilted with respect to the membrane, 3 of them "kinked" due to the presence of prolyl residues.[1]
Residues that are important for the transport mechanism are likely to be symmetrical, whereas residues involved in substrate binding will be asymmetrical reflecting the asymmetry of the substrates. By scoring the symmetry of residues in the sequence repeats, Robinson et al. (2008) identified the substrate-binding sites and salt bridge networks that are important for transport. The symmetry analyses provides an assessment of the role of residues and provides clues to the chemical identities of substrates of uncharacterized transporters.[22]
A few MC protein crystal structures are available in RCSB, including: PDB: 4C9G, 4C9H, 2BMN, 4P5X, 4P60, 4P5W
Substrates
Members of the mitochondrial carrier family are involved in transporting keto acids, amino acids, nucleotides, inorganic ions and co-factors across the mitochondrial inner membrane. The transporters are thought to share the same structural fold, which consists of six trans-membrane alpha-helices and three matrix helices, arranged with threefold pseudo-symmetry. During the transport cycle two salt bridge networks on either side of the central cavity might regulate access to a single substrate binding site in an alternating fashion. In the case of proton-substrate symporters, the substrate binding sites contain negatively charged residues that are proposed to be involved in proton transport.[23]
The transported substrates of MC family members may bind to the bottom of the cavity, and translocation results in a transient transition from a 'pit' to a 'channel' conformation.[24] An inhibitor of AAC, carboxyatractyloside, probably binds where ADP binds, in the pit on the outer surface, thus blocking the transport cycle. Another inhibitor, bongkrekic acid, is believed to stabilize a second conformation, with the pit facing the matrix. In this conformation, the inhibitor may bind to the ATP-binding site. Functional and structural roles for residues in the TMSs have been proposed.[25][26] The mitochondrial carrier signature, Px[D/E]xx[K/R], of carriers is probably involved both in the biogenesis and in the transport activity of these proteins.[27] A homologue has been identified in the mimivirus genome and shown to be a transporter for dATP and dTTP.[28]
Examples of transported compounds include:
- citrate - SLC25A1
- ornithine - SLC25A2, SLC25A15
- phosphate - SLC25A3, SLC25A23, SLC25A24, SLC25A25
- adenine nucleotide - SLC25A4, SLC25A5, SLC25A6, SLC25A31
- dicarboxylate - SLC25A10
- oxoglutarate - SLC25A11
- glutamate - SLC25A22
Examples
Human proteins containing this domain include:
- HDMCP, LOC153328, MCART1, MCART2, MCART6, MTCH1, MTCH2
- UCP1, UCP2, UCP3
- SLC25A1, SLC25A3, SLC25A4, SLC25A5, SLC25A6, SLC25A10, SLC25A11, SLC25A12, SLC25A13, SLC25A14, SLC25A16, SLC25A17, SLC25A18, SLC25A19, SLC25A21, SLC25A22, SLC25A23, SLC25A24, SLC25A25, SLC25A26, SLC25A27, SLC25A28, SLC25A29, SLC25A30, SLC25A31, SLC25A32, SLC25A33, SLC25A34, SLC25A35, SLC25A36, SLC25A37, SLC25A38, SLC25A39, SLC25A40, SLC25A41, SLC25A42, SLC25A43, SLC25A44, SLC25A45, SLC25A46
External links
- Getting a good rate of exchange – the mitochondrial ADP-ATP carrier Article at PDBe
- Transporter Classification Database - Mitochondrial Carrier Superfamily
References
- 1 2 3 "Relations between structure and function of the mitochondrial ADP/ATP carrier". Annu. Rev. Biochem. 75: 713–41. 2006. doi:10.1146/annurev.biochem.75.103004.142747. PMID 16756509.
- ↑ Kuan J, Saier MH (October 1993). "Expansion of the mitochondrial carrier family". Research in Microbiology. 144 (8): 671–2. doi:10.1016/0923-2508(93)90073-B. PMID 8140286.
- 1 2 Bamber L, Harding M, Monné M, Slotboom DJ, Kunji ER (June 2007). "The yeast mitochondrial ADP/ATP carrier functions as a monomer in mitochondrial membranes". Proceedings of the National Academy of Sciences of the United States of America. 104 (26): 10830–4. doi:10.1073/pnas.0703969104. PMC 1891095
 . PMID 17566106.
. PMID 17566106. - 1 2 Bamber L, Harding M, Butler PJ, Kunji ER (October 2006). "Yeast mitochondrial ADP/ATP carriers are monomeric in detergents". Proceedings of the National Academy of Sciences of the United States of America. 103 (44): 16224–9. doi:10.1073/pnas.0607640103. PMC 1618811
 . PMID 17056710.
. PMID 17056710. - ↑ Klingenberg M (March 1990). "Mechanism and evolution of the uncoupling protein of brown adipose tissue". Trends in Biochemical Sciences. 15 (3): 108–12. doi:10.1016/0968-0004(90)90194-G. PMID 2158156.
- ↑ Nelson DR, Lawson JE, Klingenberg M, Douglas MG (April 1993). "Site-directed mutagenesis of the yeast mitochondrial ADP/ATP translocator. Six arginines and one lysine are essential". Journal of Molecular Biology. 230 (4): 1159–70. doi:10.1006/jmbi.1993.1233. PMID 8487299.
- ↑ Jank B, Habermann B, Schweyen RJ, Link TA (November 1993). "PMP47, a peroxisomal homologue of mitochondrial solute carrier proteins". Trends in Biochemical Sciences. 18 (11): 427–8. doi:10.1016/0968-0004(93)90141-9. PMID 8291088.
- 1 2 Monné M, Palmieri F, Kunji ER (March 2013). "The substrate specificity of mitochondrial carriers: mutagenesis revisited". Molecular Membrane Biology. 30 (2): 149–59. doi:10.3109/09687688.2012.737936. PMID 23121155.
- 1 2 Dolce V, Cappello AR, Capobianco L (July 2014). "Mitochondrial tricarboxylate and dicarboxylate-tricarboxylate carriers: from animals to plants". IUBMB Life. 66 (7): 462–71. doi:10.1002/iub.1290. PMID 25045044.
- ↑ Palmieri F (June 1994). "Mitochondrial carrier proteins". FEBS Letters. 346 (1): 48–54. doi:10.1016/0014-5793(94)00329-7. PMID 8206158.
- ↑ Walker JE (1992). "The mitochondrial transporter family". Curr. Opin. Struct. Biol. 2 (4): 519–526. doi:10.1016/0959-440X(92)90081-H.
- ↑ Palmieri F (February 2004). "The mitochondrial transporter family (SLC25): physiological and pathological implications". Pflügers Archiv. 447 (5): 689–709. doi:10.1007/s00424-003-1099-7. PMID 14598172.
- ↑ Palmieri F, Rieder B, Ventrella A, Blanco E, Do PT, Nunes-Nesi A, Trauth AU, Fiermonte G, Tjaden J, Agrimi G, Kirchberger S, Paradies E, Fernie AR, Neuhaus HE (November 2009). "Molecular identification and functional characterization of Arabidopsis thaliana mitochondrial and chloroplastic NAD+ carrier proteins". The Journal of Biological Chemistry. 284 (45): 31249–59. doi:10.1074/jbc.M109.041830. PMC 2781523
 . PMID 19745225.
. PMID 19745225. - ↑ Palmieri F, Pierri CL (2010-01-01). "Mitochondrial metabolite transport". Essays in Biochemistry. 47: 37–52. doi:10.1042/bse0470037. PMID 20533899.
- 1 2 Palmieri F (2008-08-01). "Diseases caused by defects of mitochondrial carriers: a review". Biochimica et Biophysica Acta. 1777 (7-8): 564–78. doi:10.1016/j.bbabio.2008.03.008. PMID 18406340.
- ↑ Gutiérrez-Aguilar M, Baines CP (September 2013). "Physiological and pathological roles of mitochondrial SLC25 carriers". The Biochemical Journal. 454 (3): 371–86. doi:10.1042/BJ20121753. PMC 3806213
 . PMID 23988125.
. PMID 23988125. - ↑ Palmieri F (2013-06-01). "The mitochondrial transporter family SLC25: identification, properties and physiopathology". Molecular Aspects of Medicine. 34 (2-3): 465–84. doi:10.1016/j.mam.2012.05.005. PMID 23266187.
- ↑ Kuan J, Saier MH (1993-01-01). "The mitochondrial carrier family of transport proteins: structural, functional, and evolutionary relationships". Critical Reviews in Biochemistry and Molecular Biology. 28 (3): 209–33. doi:10.3109/10409239309086795. PMID 8325039.
- ↑ Endres M, Neupert W, Brunner M (June 1999). "Transport of the ADP/ATP carrier of mitochondria from the TOM complex to the TIM22.54 complex". The EMBO Journal. 18 (12): 3214–21. doi:10.1093/emboj/18.12.3214. PMC 1171402
 . PMID 10369662.
. PMID 10369662. - ↑ Ryan MT, Müller H, Pfanner N (July 1999). "Functional staging of ADP/ATP carrier translocation across the outer mitochondrial membrane". The Journal of Biological Chemistry. 274 (29): 20619–27. doi:10.1074/jbc.274.29.20619. PMID 10400693.
- ↑ Falconi M, Chillemi G, Di Marino D, D'Annessa I, Morozzo della Rocca B, Palmieri L, Desideri A (November 2006). "Structural dynamics of the mitochondrial ADP/ATP carrier revealed by molecular dynamics simulation studies". Proteins. 65 (3): 681–91. doi:10.1002/prot.21102. PMID 16988954.
- ↑ Robinson AJ, Overy C, Kunji ER (November 2008). "The mechanism of transport by mitochondrial carriers based on analysis of symmetry". Proceedings of the National Academy of Sciences of the United States of America. 105 (46): 17766–71. doi:10.1073/pnas.0809580105. PMC 2582046
 . PMID 19001266.
. PMID 19001266. - ↑ Kunji ER, Robinson AJ (August 2010). "Coupling of proton and substrate translocation in the transport cycle of mitochondrial carriers". Current Opinion in Structural Biology. 20 (4): 440–7. doi:10.1016/j.sbi.2010.06.004. PMID 20598524.
- ↑ Kunji ER, Robinson AJ (2006-10-01). "The conserved substrate binding site of mitochondrial carriers". Biochimica et Biophysica Acta. 1757 (9-10): 1237–48. doi:10.1016/j.bbabio.2006.03.021. PMID 16759636.
- ↑ Cappello AR, Curcio R, Valeria Miniero D, Stipani I, Robinson AJ, Kunji ER, Palmieri F (October 2006). "Functional and structural role of amino acid residues in the even-numbered transmembrane alpha-helices of the bovine mitochondrial oxoglutarate carrier". Journal of Molecular Biology. 363 (1): 51–62. doi:10.1016/j.jmb.2006.08.041. PMID 16962611.
- ↑ Cappello AR, Miniero DV, Curcio R, Ludovico A, Daddabbo L, Stipani I, Robinson AJ, Kunji ER, Palmieri F (June 2007). "Functional and structural role of amino acid residues in the odd-numbered transmembrane alpha-helices of the bovine mitochondrial oxoglutarate carrier". Journal of Molecular Biology. 369 (2): 400–12. doi:10.1016/j.jmb.2007.03.048. PMID 17442340.
- ↑ Zara V, Ferramosca A, Capobianco L, Baltz KM, Randel O, Rassow J, Palmieri F, Papatheodorou P (December 2007). "Biogenesis of yeast dicarboxylate carrier: the carrier signature facilitates translocation across the mitochondrial outer membrane". Journal of Cell Science. 120 (Pt 23): 4099–106. doi:10.1242/jcs.018929. PMID 18032784.
- ↑ Monné M, Robinson AJ, Boes C, Harbour ME, Fearnley IM, Kunji ER (April 2007). "The mimivirus genome encodes a mitochondrial carrier that transports dATP and dTTP". Journal of Virology. 81 (7): 3181–6. doi:10.1128/JVI.02386-06. PMC 1866048
 . PMID 17229695.
. PMID 17229695.