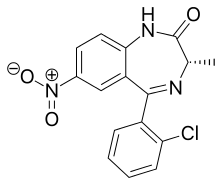
Meclonazepam
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
58662-84-3 |
| PubChem (CID) | 3033985 |
| ChemSpider |
2298544 |
| UNII |
RN43209SMA |
| ChEMBL |
CHEMBL351821 |
| Chemical and physical data | |
| Formula | C16H12ClN3O3 |
| Molar mass | 329.74 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Meclonazepam[1] ((S)-3-methylclonazepam) was discovered by a team at Hoffmann-La Roche in the 1970s and is a drug which is a benzodiazepine derivative similar in structure to clonazepam.[2] It has sedative and anxiolytic actions like those of other benzodiazepines,[3] and also has anti-parasitic effects against the parasitic worm Schistosoma mansoni.[4]
Meclonazepam was never used as medicine and instead appeared online as a designer drug.[5][6]
See also
References
- ↑ U.S. Patent 4,031,078
- ↑ The Lundbeck Institute. "Meclonazepam". Psychotropics. Lundbeck.
- ↑ Ansseau, M.; Doumont, A.; Thiry, D.; Von Frenckell, R.; Collard, J. (1985). "Initial study of methylclonazepam in generalized anxiety disorder. Evidence for greater power in the cross-over design". Psychopharmacology. 87 (2): 130–135. doi:10.1007/bf00431795. PMID 3931136.
- ↑ O'Boyle, C.; Lambe, R.; Darragh, A. (1985). "Central Effects in Man of the Novel Schistosomicidal Benzodiazepine Meclonazepam". European Journal of Clinical Pharmacology. 29 (1): 105–108. doi:10.1007/bf00547377. PMID 4054198.
- ↑ Markus R. Meyer; Madeleine Pettersson Bergstrand; Anders Helander; Olof Beck (May 2016). "Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes". Analytical and Bioanalytical Chemistry. 408 (13): 3571–3591. doi:10.1007/s00216-016-9439-6. PMID 27071765.
- ↑ Pettersson Bergstrand M, Helander A, Hansson T, Beck O (2016). "Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays". Drug Test Anal. doi:10.1002/dta.2003. PMID 27366870.
Further reading
- Abdul-Ghani, R. A.; Loutfy, N.; Hassan, A. (2009). "Experimentally promising antischistosomal drugs: A review of some drug candidates not reaching the clinical use". Parasitology Research. 105 (4): 899–906. doi:10.1007/s00436-009-1546-2. PMID 19588166.
This article is issued from Wikipedia - version of the 10/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.