Marsh test
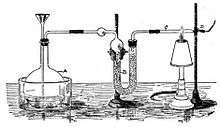
The Marsh test is a highly sensitive method in the detection of arsenic, especially useful in the field of forensic toxicology when arsenic was used as a poison. It was developed by the chemist James Marsh and first published in 1836.[1][2]
Arsenic, in the form of white arsenic trioxide As2O3, was a highly favored poison, for it is odorless, easily incorporated into food and drink, and before the advent of the Marsh test, untraceable in the body. In France, it came to be known as poudre de succession ("inheritance powder"). For the untrained, arsenic poisoning would have symptoms similar to cholera.
Precursor methods
The first breakthrough in the detection of arsenic poisoning was in 1775 when Carl Wilhelm Scheele discovered a way to change arsenic trioxide to garlic-smelling arsine gas (AsH3), by treating it with nitric acid (HNO3) and combining it with zinc.[3]
- As2O3 + 6 Zn + 12 HNO3 → 2 AsH3 + 6 Zn(NO3)2 + 3 H2O
In 1787, German physician Johann Metzger (1739-1805) discovered that if arsenic trioxide were heated in the presence of carbon, the arsenic would sublimate.[4] This is the reduction of As2O3 by carbon:
- 2 As2O3 + 3 C → 3 CO2 + 4 As
In 1806, Valentin Rose took the stomach of a victim suspected of being poisoned and treated it with potassium carbonate (K2CO3), calcium oxide (CaO) and nitric acid.[5] Any arsenic present would appear as arsenic trioxide and then could be subjected to Metzger's test.
However, the most common test (and used even today in water test kits) was discovered by Samuel Hahnemann. It would involve combining a sample fluid with hydrogen sulfide (H2S) in the presence of hydrochloric acid (HCl). A yellow precipitate, arsenic trisulfide (As2S3) would be formed if arsenic was present.
Circumstances and methodology
Even so, these tests have proven not to be sensitive enough. In 1832, a certain John Bodle was brought to trial for poisoning his grandfather by putting arsenic in his coffee. James Marsh, a chemist working at the Royal Arsenal in Woolwich was called by the prosecution to try to detect its presence. He performed the standard test by passing hydrogen sulfide through the suspect fluid. While Marsh was able to detect arsenic, the yellow precipitate did not keep very well, and by the time it was presented to the jury it deteriorated. The jury was not convinced, and John Bodle was acquitted.
Angered and frustrated by this, especially when John Bodle confessed later that he indeed killed his grandfather, Marsh decided to devise a better test to demonstrate the presence of arsenic. Taking Scheele's work as a basis, he constructed a simple glass apparatus capable of not only detecting minute traces of arsenic but also measuring its quantity. Adding a sample of tissue or body fluid to a glass vessel with zinc and acid would produce arsine gas if arsenic was present, in addition to the hydrogen that would be produced regardless by the zinc reacting with the acid. Igniting this gas mixture would oxidize any arsine present into arsenic and water vapor. This would cause a cold ceramic bowl held in the jet of the flame to be stained with a silvery-black deposit of arsenic, physically similar to the result of Metzger's reaction. The intensity of the stain could then be compared to films produced using known amounts of arsenic.[6] Not only could minute amounts of arsenic be detected (as little as 0.02 mg), the test was very specific for arsenic. Although antimony (Sb) could give a false-positive test by forming a similar black deposit, it would not dissolve in a solution of sodium hypochlorite (NaOCl), while arsenic would.
Specific reactions involved
The Marsh test treats the sample with sulphuric acid and arsenic-free zinc. Even if there are minute amounts of arsenic present, the zinc reduces the trivalent arsenic (As3+ ). Here are the two half-reactions:
- Oxidation: Zn → Zn2+ + 2 e−
- Reduction: As2O3 + 12 e− + 6 H+ → 2 As3− + 3 H2O
Overall, we have this reaction:
- As2O3 + 6 Zn + 6 H+ → 2 As3− + 6 Zn2+ + 3 H2O
In an acidic medium, As3−
is protonated to form arsine gas (AsH3), so adding sulphuric acid (H2SO4) to each side of the equation we get:
- As2O3 + 6 Zn + 6 H+ + 6 H2SO4 → 2 As3− + 6 H2SO4 + 6 Zn2+ + 3 H2O
As the As3− combines with the H+ to form arsine:
- As2O3 + 6 Zn + 6 H+ + 6 H2SO4 → 2 AsH3 + 6 ZnSO4 + 3 H2O + 6 H+
By eliminating the common ions:
- As2O3 + 6 Zn + 6 H2SO4 → 2 AsH3 + 6 ZnSO4 + 3 H2O
First notable application
Although the Marsh test was efficacious, its first publicly documented use—in fact, the first time evidence from forensic toxicology was ever introduced—was in Tulle, France in 1840 with the celebrated LaFarge poisoning case. Charles LaFarge, a foundry owner, was suspected of being poisoned with arsenic by his wife Marie. The circumstantial evidence was great: it was shown that she bought arsenic trioxide from a local chemist, supposedly to kill rats which infested their home. In addition, their maid swore that she had mixed a white powder into his drink. Although the food was found to be positive for the poison using the old methods as well as the Marsh test, when the husband's body was exhumed and tested, the chemists assigned to the case were not able to detect arsenic. Mathieu Orfila, the renowned toxicologist retained by the defense and an acknowledged authority of the Marsh test examined the results. He performed the test again and demonstrated that the Marsh test was not at fault for the misleading results but rather those who performed it did it incorrectly. Orfila thus proved the presence of arsenic in LaFarge's body using the test. As a result of this, Marie was found guilty and sentenced to life imprisonment.
Effects
The case proved to be controversial, for it divided the country into factions who were convinced or otherwise of Mme. LaFarge's guilt; nevertheless, the impact of the Marsh test was great. The French press covered the trial and gave the test the publicity it needed to give the field of forensic toxicology the legitimacy it deserved, although in some ways it trivialized it: Marsh test assays were actually done in salons, public lectures and even in some plays that recreated the LaFarge case.
The existence of the Marsh test also served a deterrent effect: deliberate arsenic poisonings became rarer because of the fear of discovery became more prevalent.
See also
References
- ↑ Marsh J. (1836). "Account of a method of separating small quantities of arsenic from substances with which it may be mixed". Edinburgh New Philosophical Journal. 21: 229–236.
- ↑ Marsh test[J. 2013.]
- ↑ Scheele, Carl Wilhelm (1775) "Om Arsenik och dess syra" (On arsenic and its acid), Kongliga Vetenskaps Academiens Handlingar (Proceedings of the Royal Scientific Academy [of Sweden]), 36 : 263-294. From p. 290: "Med Zinck. 30. (a) Denna år den endaste af alla så hela som halfva Metaller, som i digestion met Arsenik-syra effervescerar." (With zinc. 30. (a) This is the only [metal] of all whole- as well as semi-metals that effervesces on digestion with arsenic acid.) Scheele collected the arsine and put a mixture of arsine and air into a cylinder. From p. 291: "3:0, Då et tåndt ljus kom når o̊pningen, tåndes luften i kolfven med en småll, lågan for mot handen, denna blef o̊fvedragen med brun fårg, … " (3:0, Then as [the] lit candle came near the opening [of the cylinder], the gases in [the] cylinder ignited with a bang ; [the] flame [rushed] towards my hand, which became coated with [a] brown color, … )
- ↑ Metzger, Johann Daniel, Kurzgefasstes System der gerichtlichen Arzneiwissenschaft (Concise system of forensic medicine), 2nd ed. (Königsberg and Leipzig, (Germany): Goebbels und Unzer, 1805), pp. 238–239. In a footnote on p. 238, Metzger mentions that if a sample that's suspected of containing arsenic trioxide (Arsenik) is heated on a copper plate (Kupferblech), then, when arsenic vapor lands on the plate, it will condense to form a shiny silver-white (weisse Silberglanz) patch. He also mentions that if a sample containing arsenic trioxide is large enough, metallic arsenic can be produced from it. From the footnote on p. 239: "b) Am besten geschieht sie, wenn mann den Arsenik mit einem fetten Oel zum Brey macht und in einer Retorte so lange distillirt, bis keine ölichte Dämpfe mehr übergehen, dann aber das Feuer verstärkt, wodurch der Arsenik - König sich sublimirt." ( b) It's best of all when one makes a paste of the arsenic trioxide with a fatty oil and distills it in a retort long enough until no more oily vapors pass over [and] then one intensifies the fire, whereby [metallic] arsenic is sublimated.)
- ↑ Valentin Rose (1806) "Ueber das zweckmäßigste Verfahren, um bei Vergiftungen mit Arsenik letzern aufzufinden und darzustellen" (On the most effective method, in cases of poisoning with arsenic, to discover and show the latter), Journal für Chemie und Physik, 2 : 665-671.
- ↑ http://www.chm.bris.ac.uk/motm/arsine/arsineh.htm
- Marsh J. (1837). "Arsenic; nouveau procédé pour le découvrir dans les substances auxquelles il est mêlé". Journal de Pharmacie. 23: 553–562.
- Marsh, James (1837). "Beschreibung eines neuen Verfahrens, um kleine Quantitäten Arsenik von den Substanzen abzuscheiden, womit er gemischt ist". Liebigs Annalen der Chemie. 23 (2): 207. doi:10.1002/jlac.18370230217.
- Mohr C. F. (1837). "Zusätze zu der von Marsh angegebenen Methode, den Arsenik unmittelbar im regulinischen Zustande aus jeder Flüssigkeit auszuscheiden" [Addenda to the method given by Marsh for separating arsenic immediately in the metallic state from any liquid]. Annalen der Chemie und Pharmacie. 23 (2): 217–225. doi:10.1002/jlac.18370230218.
- Lockemann, Georg (1905). "Über den Arsennachweis mit dem Marshschen Apparate" [On the detection of arsenic with the Marsh apparatus]. Angewandte Chemie. 18 (11): 416. doi:10.1002/ange.19050181104.
- Harkins, W. D. (1910). "The Marsh test and Excess Potential (First Paper.1) The Quantitative Determination of Arsenic". Journal of the American Chemical Society. 32 (4): 518–530. doi:10.1021/ja01922a008.
- Campbell W. A. (1965). "Some landmarks in the history of arsenic testing". Chemistry in Britain. 1: 198–202.
External links
- McMuigan, Hugh (1921). An Introduction to Chemical Pharmacology. Philadelphia: P. Blakiston's Son & Co. pp. 396–397. Retrieved 2007-12-16.
- Wanklyn, James Alfred (1901). Arsenic. London: Kegan Paul, Trench, Trübner & Co. Ltd. pp. 39–57. Retrieved 2007-12-16.