Low-density lipoprotein receptor gene family
| Low-density lipoprotein receptor domain class A | |||||||||
|---|---|---|---|---|---|---|---|---|---|
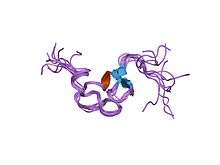 Structure of a cysteine-rich repeat from the low-density lipoprotein receptor.[1] | |||||||||
| Identifiers | |||||||||
| Symbol | Ldl_recept_a | ||||||||
| Pfam | PF00057 | ||||||||
| InterPro | IPR002172 | ||||||||
| SMART | SM00192 | ||||||||
| PROSITE | PS50068 | ||||||||
| SCOP | 1ldl | ||||||||
| SUPERFAMILY | 1ldl | ||||||||
| |||||||||
| Low-density lipoprotein receptor domain class B | |||||||||
|---|---|---|---|---|---|---|---|---|---|
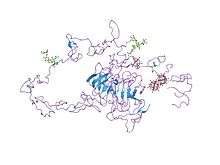 Structure of the LDL receptor extracellular domain at endosomal pH.[2] | |||||||||
| Identifiers | |||||||||
| Symbol | Ldl_recept_b | ||||||||
| Pfam | PF00058 | ||||||||
| Pfam clan | 6CL0186 | ||||||||
| InterPro | IPR000033 | ||||||||
| SMART | SM00135 | ||||||||
| PROSITE | PS51120 | ||||||||
| SCOP | 1lrx | ||||||||
| SUPERFAMILY | 1lrx | ||||||||
| |||||||||
The low-density lipoprotein receptor gene family codes for a class of structurally related cell surface receptors that fulfill diverse biological functions in different organs, tissues, and cell types.[3] The role that is most commonly associated with this evolutionarily ancient family is cholesterol homeostasis (maintenance of appropriate concentration of cholesterol). In humans, excess cholesterol in the blood is captured by low-density lipoprotein (LDL) and removed by the liver via endocytosis of the LDL receptor.[4] Recent evidence indicates that the members of the LDL receptor gene family are active in the cell signalling pathways between specialized cells in many, if not all, multicellular organisms.[5][6]
There are seven members of the LDLR family in mammals, namely:
- LDLR
- VLDL receptor (VLDLR)
- ApoER2, or LRP8
- Low density lipoprotein receptor-related protein 4
- also known as multiple epidermal growth factor (EGF) repeat-containing protein (MEGF7)
- LDLR-related protein 1
- LDLR-related protein 1b
- Megalin.
Human proteins containing this domain
Listed below are human proteins containing low-density lipoprotein receptor domains:
Class A
C6; C7; 8A; 8B; C9; CD320; CFI; CORIN; DGCR2; HSPG2; LDLR; LDLRAD2; LDLRAD3; LRP1; LRP10; LRP11; LRP12; LRP1B; LRP2; LRP3; LRP4; LRP5; LRP6; LRP8; MAMDC4; MFRP; PRSS7; RXFP1; RXFP2; SORL1; SPINT1; SSPO; ST14; TMPRSS4; TMPRSS6; TMPRSS7; TMPRSS9 (serase-1B); VLDLR;
Class B
EGF; LDLR; LRP1; LRP10; LRP1B; LRP2; LRP4; LRP5; LRP5L; LRP6; LRP8; NID1; NID2; SORL1; VLDLR;
See also
- Soluble low-density lipoprotein receptor-related protein (sLRP) - impaired function is related to Alzheimer's Disease.
Structure
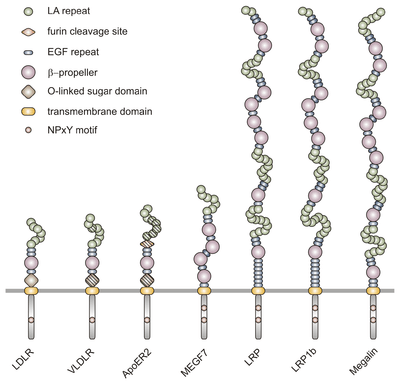
The members of the LDLR family are characterized by distinct functional domains present in characteristic numbers. These modules are:
- LDL receptor type A (LA) repeats of 40 residues each, displaying a triple-disulfide-bond-stabilized negatively charged surface; certain head-to-tail combinations of these repeats are believed to specify ligand interactions;
- LDL receptor type B repeats, also known as EGF precursor homology regions, containing EGF-like repeats and YWTD beta propeller domains;
- a transmembrane domain, and
- the cytoplasmic region with (a) signal(s) for receptor internalization via coated pits, containing the consensus tetrapeptide Asn-Pro-Xaa-Tyr (NPxY). This cytoplasmic tail controls both endocytosis and signaling by interacting with the phosphotyrosine binding (PTB) domain-containing proteins.
In addition to these domains which can be found in all receptors of the gene family, LDL receptor and certain isoforms of ApoER2 and VLDLR contain a short region which can undergo O-linked glycosylation, known as O-linked sugar domain. ApoER2 moreover, can harbour a cleavage site for the protease furin between type A and type B repeats which enables production of a soluble receptor fragment by furin-mediated processing.
References
- ↑ Daly NL, Scanlon MJ, Djordjevic JT, Kroon PA, Smith R (July 1995). "Three-dimensional structure of a cysteine-rich repeat from the low-density lipoprotein receptor". Proc. Natl. Acad. Sci. U.S.A. 92 (14): 6334–8. doi:10.1073/pnas.92.14.6334. PMC 41512
 . PMID 7603991.
. PMID 7603991. - ↑ Rudenko G, Henry L, Henderson K, et al. (December 2002). "Structure of the LDL receptor extracellular domain at endosomal pH". Science. 298 (5602): 2353–8. doi:10.1126/science.1078124. PMID 12459547.
- ↑ Nykjaer A, Willnow TE (June 2002). "The low-density lipoprotein receptor gene family: a cellular Swiss army knife?". Trends Cell Biol. 12 (6): 273–80. doi:10.1016/S0962-8924(02)02282-1. PMID 12074887.
- ↑ Li Y, Lu W, Marzolo MP, Bu G (May 2001). "Differential functions of members of the low density lipoprotein receptor family suggested by their distinct endocytosis rates". J. Biol. Chem. 276 (21): 18000–6. doi:10.1074/jbc.M101589200. PMID 11279214.
- ↑ Gotthardt M, Trommsdorff M, Nevitt MF, Shelton J, Richardson JA, Stockinger W, Nimpf J, Herz J (August 2000). "Interactions of the low density lipoprotein receptor gene family with cytosolic adaptor and scaffold proteins suggest diverse biological functions in cellular communication and signal transduction". J. Biol. Chem. 275 (33): 25616–24. doi:10.1074/jbc.M000955200. PMID 10827173.
- ↑ Beffert U, Stolt PC, Herz J (March 2004). "Functions of lipoprotein receptors in neurons". J. Lipid Res. 45 (3): 403–9. doi:10.1194/jlr.R300017-JLR200. PMID 14657206.
External links
- Schematic representation of the seven mammalian LDL receptor (LDLR) family members
- LDL receptor family members
- LDL Receptors at the US National Library of Medicine Medical Subject Headings (MeSH)