Lithium oxide
 | |
| Names | |
|---|---|
| IUPAC name
Lithium oxide | |
| Other names
Kickerite | |
| Identifiers | |
| 12057-24-8 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 145811 |
| ECHA InfoCard | 100.031.823 |
| PubChem | 166630 |
| RTECS number | OJ6360000 |
| |
| |
| Properties | |
| Li 2O | |
| Molar mass | 29.88 g/mol |
| Appearance | white solid |
| Density | 2.013 g/cm3 |
| Melting point | 1,438 °C (2,620 °F; 1,711 K) |
| Boiling point | 2,600 °C (4,710 °F; 2,870 K) |
| reacts violently to form LiOH | |
| log P | 9.23 |
| Refractive index (nD) |
1.644 [1] |
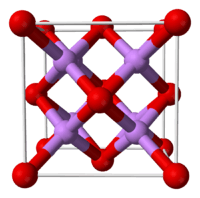
| Structure | |
| Antifluorite (cubic), cF12 | |
| Fm3m, No. 225 | |
| Tetrahedral (Li+); cubic (O2−) | |
| Thermochemistry | |
| 1.8105 J/g K or 54.1 J/mol K | |
| Std molar entropy (S |
37.89 J/mol K |
| Std enthalpy of formation (ΔfH |
-20.01 kJ/g or -595.8 kJ/mol |
| Gibbs free energy (ΔfG˚) |
-562.1 kJ/mol |
| Hazards | |
| Main hazards | Corrosive, reacts violently with water |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Lithium sulfide |
| Other cations |
Sodium oxide Potassium oxide Rubidium oxide Caesium oxide |
| Lithium peroxide Lithium superoxide | |
| Related compounds |
Lithium hydroxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lithium oxide (Li
2O) or lithia is an inorganic chemical compound. Lithium oxide is formed along with small amounts of lithium peroxide when lithium metal is burned in the air and combines with oxygen:[2]
- 4Li + O
2 → 2Li
2O.
Pure Li
2O can be produced by the thermal decomposition of lithium peroxide, Li
2O
2, at 450 °C[2]
- 2Li
2O
2 → 2Li
2O + O
2
Structure
In the solid state lithium oxide adopts an antifluorite structure which is related to the CaF
2, fluorite structure with Li cations substituted for fluoride anions and oxide anions substituted for calcium cations.[3]
The ground state gas phase Li
2O molecule is linear with a bond length consistent with strong ionic bonding.[4][5] VSEPR theory would predict a bent shape similar to H
2O.
Uses
Lithium oxide is used as a flux in ceramic glazes; and creates blues with copper and pinks with cobalt. Lithium oxide reacts with water and steam, forming lithium hydroxide and should be isolated from them.
Its usage is also being investigated for non-destructive emission spectroscopy evaluation and degradation monitoring within thermal barrier coating systems. It can be added as a co-dopant with yttria in the zirconia ceramic top coat, without a large decrease in expected service life of the coating. At high heat, lithium oxide emits a very detectable spectral pattern, which increases in intensity along with degradation of the coating. Implementation would allow in situ monitoring of such systems, enabling an efficient means to predict lifetime until failure or necessary maintenance.
Lithium metal might be obtained from lithium oxide by electrolysis, releasing oxygen as by-product.
See also
References
- ↑ Pradyot Patnaik. Handbook of Inorganic Chemicals. McGraw-Hill, 2002, ISBN 0-07-049439-8
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1984). Chemistry of the Elements. Oxford: Pergamon Press. pp. 97–99. ISBN 0-08-022057-6.
- ↑ Zintl, E.; Harder, A.; Dauth B. (1934). "Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums". Zeitschrift für Elektrochemie und Angewandte Physikalische Chemie. 40: 588–93.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ A spectroscopic determination of the bond length of the LiOLi molecule: Strong ionic bonding, D. Bellert, W. H. Breckenridge, J. Chem. Phys. 114, 2871 (2001); doi:10.1063/1.1349424