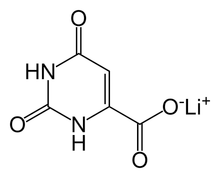
Lithium orotate
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| CAS Number |
5266-20-6 |
| PubChem (CID) | 23686432 |
| ChemSpider |
71254 |
| ECHA InfoCard | 100.023.711 |
| Chemical and physical data | |
| Formula | C5H3LiN2O4 |
| Molar mass | 162.03 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Lithium orotate, is a salt of orotic acid and lithium. It is available as the monohydrate, LiC5H3N2O4·H2O.[1] In this compound, lithium is non-covalently bound to an orotate ion, rather than to a carbonate or other ion. It is marketed as a dietary supplement, though only barely researched between 1973-1986 to treat certain medical conditions, such as alcoholism,[2] and Alzheimer's disease.
While lithium orotate is capable of providing lithium to the body, like lithium carbonate and other lithium salts, there are no systematic reviews supporting the efficacy of lithium orotate and it is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of any medical condition.
Effectiveness
In 1973, Nieper reported that lithium orotate contained 3.83 mg of elemental lithium per 100 mg and lithium carbonate contained 18.8 mg of elemental lithium per 100 mg[3] Nieper went on to claim that lithium did not dissolve from the orotate carrier until it passed through the blood–brain barrier; however, a 1976 study documented that lithium concentrations within the brains of rats were not statistically different between equivalent dosages of lithium from lithium orotate, lithium carbonate, or lithium chloride.[4] While this study was conducted with rats, it directly contradicts the aforementioned assumptions made by Nieper and others. The pharmacokinetics of lithium orotate in human brains is poorly documented and further inquiry is needed to affirm that lithium concentrations in the brain are higher with lithium orotate. Major medical research has not been conducted on lithium orotate since the 1980s due to its patent status and the abundant availability of lithium carbonate. As previously stated, lithium intake appears to be effective even at low doses, and this may account for lithium orotates claimed effectiveness.[5][6][7]
Safety
A 1979 murine study states "The renal lithium clearance was significantly lower, the kidney weight and the lithium concentrations in serum, kidney and heart significantly higher after injection of lithium orotate than after injection of lithium carbonate".[8] That study also concluded, "This data suggest the possibility that lower doses of lithium orotate than lithium carbonate may achieve therapeutic brain lithium concentrations and relatively stable serum concentrations."
Orotic acid can be mutagenic in mammalian somatic cells. It is also mutagenic for bacteria and yeast.[9]
See also
References
- ↑ Ina Bach; Otto Kumberger; Hubert Schmidbaur (1990). "Orotate complexes. Synthesis and crystal structure of lithium orotate( - I) monohydrate and magnesium bis[ orotate( - I)] octahydrate". Chem. Ber. 123 (12): 2267–2271. doi:10.1002/cber.19901231207.
- ↑ Sartori HE (1986). "Lithium orotate in the treatment of alcoholism and related conditions". Alcohol. 3 (2): 97–100. doi:10.1016/0741-8329(86)90018-2. PMID 3718672.
- ↑ Nieper, Hans Alfred (1973), "The clinical applications of lithium orotate. A two years study", Agressologie., Masson, Proquest, 14 (6): 407–11, ISSN 0002-1148, PMID 4607169
- ↑ Smith DF (April 1976). "Lithium orotate, carbonate, and chloride: pharmacokinetics, polydipsia and polyuria in rats". Br J Pharmacol. 56 (4): 399–402. doi:10.1111/j.1476-5381.1976.tb07449.x. PMC 1666891
 . PMID 1260219.
. PMID 1260219. - ↑ Alevizos B, Alevizos E, Leonardou A, Zervas I (2012). "Low dosage lithium augmentation in venlafaxine resistant depression: An open-label study". Psychiatrike. 23 (2): 143–8. PMID 22796912.
- ↑ Nunes MA, Viel TA, Buck HS I (2013). "Microdose lithium treatment stabilized cognitive impairment in patients with Alzheimer's disease". Curr Alzheimer Res. 10 (1): 104–7. doi:10.2174/156720513804871354. PMID 22746245.
- ↑ Berger GE, Wood SJ, Ross M, Hamer CA, Wellard RM, Pell G, Phillips L, Nelson B, Amminger GP, Yung AR, Jackson G, Velakoulis D, Pantelis C, Manji H, McGorry PD I (2012). "Neuroprotective effects of low-dose lithium in individuals at ultra-high risk for psychosis. A longitudinal MRI/MRS study". Curr Pharm Des. 18 (4): 570–5. doi:10.2174/138161212799316163. PMID 22239590.
- ↑ Smith DF, Schou M (March 1979). "Kidney function and lithium concentrations of rats given an injection of lithium orotate or lithium carbonate". J. Pharm. Pharmacol. 31 (3): 161–3. doi:10.1111/j.2042-7158.1979.tb13461.x. PMID 34690.
- ↑ Orotic acid MSDS