List of refractive indices
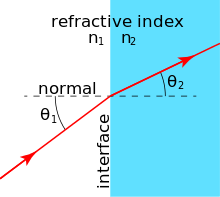
Many materials have a well-characterized refractive index, but these indices depend strongly upon the frequency of light. Standard refractive index measurements are taken at the "yellow doublet" sodium D line, with a wavelength of 589 nanometers.
There are also weaker dependencies on temperature, pressure/stress, etc., as well on precise material compositions (presence of dopants, etc.); for many materials and typical conditions, however, these variations are at the percent level or less. Thus, it is especially important to cite the source for an index measurement if precision is required.
In general, an index of refraction is a complex number with both a real and imaginary part, where the latter indicates the strength of absorption loss at a particular wavelength—thus, the imaginary part is sometimes called the extinction coefficient . Such losses become particularly significant, for example, in metals at short (e.g. visible) wavelengths, and must be included in any description of the refractive index.
List
| Material | λ (nm) | n | Ref. |
|---|---|---|---|
| Vacuum | 1 (by definition) | ||
| Air at STP | 1.000277 | ||
| Gases at 0 °C and 1 atm | |||
| Air | 529.29 | 1.000293 | [1] |
| Carbon dioxide | 589.29 | 1.001 | [2][3][4] |
| Helium | 589.29 | 1.000036 | [1] |
| Hydrogen | 589.29 | 1.000132 | [1] |
| Liquids at 20 °C | |||
| Arsenic trisulfide and sulfur in methylene iodide | 1.9 | [5] | |
| Benzene | 589.29 | 1.501 | [1] |
| Carbon disulfide | 589.29 | 1.628 | [1] |
| Carbon tetrachloride | 589.29 | 1.461 | [1] |
| Ethanol (ethyl alcohol) | 589.29 | 1.361 | [1] |
| Silicone oil | 1.336–1.582 | [6] | |
| Water | 589.29 | 1.330 | [1] |
| 10% Glucose solution in water | 589.29 | 1.3477 | [7] |
| 20% Glucose solution in water | 589.29 | 1.3635 | [7] |
| 60% Glucose solution in water | 589.29 | 1.4394 | [7] |
| Solids at room temperature | |||
| Titanium dioxide (rutile phase) | 589.29 | 2.614 | [8][9] |
| Diamond | 589.29 | 2.419 | [1] |
| Silicon carbide (Moissanite) | 2.65–2.69 | ||
| Strontium titanate | 589.29 | 2.41 | [10] |
| Amber | 589.29 | 1.55 | [1] |
| Fused silica (a pure form of glass, also called fused quartz) | 589.29 | 1.458 | [1][11] |
| Sodium chloride | 589.29 | 1.544 | [12] |
| Other materials | |||
| Liquid helium | 1.025 | ||
| Water ice | 1.31 | ||
| TFE/PDD (Teflon AF) | 1.315 | [13][14] | |
| Cryolite | 1.338 | ||
| Cytop | 1.34 | [15] | |
| Acetone | 1.36 | ||
| Ethanol | 1.36 | ||
| Polytetrafluoroethylene (Teflon) | 1.35–1.38 | [16] | |
| Sugar solution, 25% | 1.3723 | [17] | |
| Cornea (human) | 1.373/1.380/1.401 | [18] | |
| Lens (human) | 1.386–1.406 | ||
| Liver (human) | 964 | 1.369 | [19] |
| Intestinal mucosa (human) | 964 | 1.329-1.338 | [20] |
| Kerosene | 1.39 | ||
| Sylgard 184 (polydimethylsiloxane) | 1.4118 | [21] | |
| Sugar solution, 50% | 1.4200 | [17] | |
| Polylactic acid | 1.46 | [22] | |
| Pyrex (a borosilicate glass) | 1.470 | [23] | |
| Glycerol | 1.4729 | ||
| Sugar solution, 75% | 1.4774 | [17] | |
| Poly(methyl methacrylate) (PMMA) | 1.4893–1.4899 | ||
| Acrylic glass | 1.490–1.492 | ||
| Halite (rock salt) | 1.516 | ||
| Crown glass (pure) | 1.50–1.54 | ||
| PETg | 1.57 | ||
| Polyethylene terephthalate (PET) | 1.5750 | ||
| Polycarbonate | 0.15e9 | 1.60 | [24] |
| Crown glass (impure) | 1.485–1.755 | ||
| Flint glass (pure) | 1.60–1.62 | ||
| Bromine | 1.661 | ||
| Flint glass (impure) | 1.523–1.925 | ||
| Sapphire | 1.762–1.778 | ||
| Boron nitride | 2-2.14 | [25] | |
| Cubic zirconia | 2.15–2.18 | [26] | |
| Potassium niobate (KNbO3) | 2.28 | ||
| Zinc oxide | 390 | 2.4 | |
| Cinnabar (mercury sulfide) | 3.02 | ||
| Silicon | 1200 - 8500 | 3.42–3.48 | [27] |
| Gallium(III) phosphide | 3.5 | ||
| Gallium(III) arsenide | 3.927 | ||
| Germanium | 3000 - 16000 | 4.05–4.01 | [28] |
See also
- Sellmeier equation
- Corrective lens#Ophthalmic material property tables
- Optical properties of water and ice
References
- 1 2 3 4 5 6 7 8 9 10 11 Zajac, Alfred; Hecht, Eugene (18 March 2003). Optics, Fourth Edition. Pearson Higher Education. ISBN 978-0-321-18878-6.
- ↑ Morgan, Joseph (1953). Introduction to Geometrical and Physical Optics. McGraw-Hill Book Company, INC.
- ↑ Hodgman, Charles D. (1957). Handbook of Chemistry and Physics. Chemical Rubber Publishing Co.
- ↑ Pedrotti, Frank L.; Pedrotti, Leno M.; Pedrotti, Leno S. (2007). Introduction to Optics, Third Edition. Pearson Prentice Hall. p. 221. ISBN 0-13-149933-5.
- ↑ Meyrowitz, R, A compilation and classification of immersion media of high index of refraction, American Mineralogist 40: 398 (1955)
- ↑ "Silicone Fluids: Stable and Inert Material" (PDF). Gelest, Inc. 1998.
- 1 2 3 Lide, David R. Lide, ed. (2001). CRC Handbook of Physics and Chemistry (82nd ed.). Cleveland, OH: The Chemical Rubber Company. ISBN 0-8493-0482-2.
- ↑ Polyanskiy, Mikhail N. "Optical constants of TiO2 (Titanium dioxide)". Refractive Index Database.
- ↑ Shannon, Robert D.; Shannon, Ruth C.; Medenbach, Olaf; Fischer, Reinhard X. (25 Oct 2002). "Refractive Index and Dispersion of Fluorides and Oxides" (PDF). J. Phys. Chem. Ref. Data. American Institute of Physics. 31 (4): 931–970.
- ↑ Frye, Asa; French, R. H.; Bonnell, D. A. (2003). "Optical properties and electronic structure of oxidized and reduced single-crystal strontium titanate" (PDF). Zeitschrift für Metallkunde. 94 (3): 226. doi:10.3139/146.030226. Retrieved 11 July 2014.
- ↑ Tan, G; Lemon, M.; Jones, D.; French, R. (2005). "Optical properties and London dispersion interaction of amorphous and crystalline {SiO2} determined by vacuum ultraviolet spectroscopy and spectroscopic ellipsometry" (PDF). Physical Review B. 72 (20). Bibcode:2005PhRvB..72t5117T. doi:10.1103/PhysRevB.72.205117. Retrieved 11 July 2014.
- ↑ Serway, Raymond A.; Faughn, Jerry S. (2003). College Physics, 6th Edition. Brooks/Cole. p. 692. ISBN 978-0-03-035114-3.
- ↑ "Teflon AF". Retrieved 2010-10-14.
- ↑ Yang, Min K. (July 2008). "Optical properties of Teflon® {AF} amorphous fluoropolymers" (PDF). Journal of Micro/Nano Lithography. 7 (3): 033010. doi:10.1117/1.2965541. Retrieved 11 July 2014.
- ↑ "CYTOP Amorphous Fluoropolymer". AGCCE Chemicals Europe, Ltd. Retrieved 2010-10-14.
- ↑ French, Roger H.; Rodriguez-Parada, J. M.; Yang, M. K.; et al. (2009). "Optical properties of materials for concentrator photovoltaic systems" (PDF). IEEE Photovoltaic Specialists Conference: 000394. doi:10.1109/PVSC.2009.5411657. ISBN 978-1-4244-2949-3. Retrieved 11 July 2014.
- 1 2 3 "Manual for Sugar Solution Prism" (PDF). A/S S. Frederiksen. 03.08.05. Retrieved 2012-03-21. Check date values in:
|date=(help) - ↑ Patel, S; Marshall, J; Fitzke, FW 3rd. (Mar–Apr 1995). "Refractive index of the human corneal epithelium and stroma". J Refract Surg. 11 (2): 100–105. PMID 7634138.
- ↑ Giannios, P; et, al (2016). "Visible to near-infrared refractive properties of freshly-excised human-liver tissues: marking hepatic malignancies". Sci. Rep. 6: 27910. doi:10.1038/srep27910.
- ↑ Giannios, P; al, et (2016). "Complex refractive index of normal and malignant human colorectal tissue". J. Biophotonics. doi:10.1002/jbio.201600001.
- ↑ "184 Silicone Elastomer" (PDF) (Product Information). Dow Corning. Retrieved 2012-12-11.
- ↑ Gonçalves, Carla M. B.; Coutinho, Joa˜o A. P.; Marrucho, Isabel M. (2010). "Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; Chapter 8: Optical Properties". p. 97. doi:10.1002/9780470649848.ch8. ISBN 9780470649848. Retrieved 2012-10-25.
|chapter=ignored (help) - ↑ University of Liverpool. "Absolute Refractive Index". Materials Teaching Educational Resources. MATTER Project. Retrieved 2007-10-18.
- ↑ C. R. Garcia, J. Correa, D. Espalin, J. H. Barton, R. C. Rumpf, R. Wicker, V. Gonzalez, "3D Printing of Anisotropic Metamaterials," PIER Lett, Vol. 34, pp. 75-82, 2012.
- ↑ "Combat Boron Nitride" (PDF). Saint Gobain. Retrieved 2016-06-12.
- ↑ French, Roger H.; Glass, S.; Ohuchi, F.; et al. (1994). "Experimental and theoretical determination of the electronic structure and optical properties of three phases of {ZrO2}" (PDF). Physical Review B. 49 (8): 5133. Bibcode:1994PhRvB..49.5133F. doi:10.1103/PhysRevB.49.5133. Retrieved 11 July 2014.
- ↑ "Silicon". Pmoptics.com. Retrieved 2014-08-21.
- ↑ "Germanium". Pmoptics.com. Retrieved 2014-08-21.
External links
- International Association for the Properties of Water and Steam
- Ioffe institute, Russian Federation
- Crystran, United Kingdom
- Jena University, Germany
- Hyperphysics list of refractive indices
- Luxpop: Index of refraction values and photonics calculations
- Kaye and Laby Online Provided by the National Physical Laboratory, UK
- List of Refractive Indices of Solvents