Lipofuscin
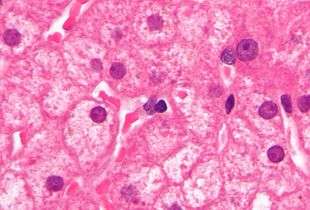
Lipofuscin is the name given to finely granular yellow-brown pigment granules[1] composed of lipid-containing residues of lysosomal digestion. It is considered to be one of the aging or "wear-and-tear" pigments, found in the liver, kidney, heart muscle, retina, adrenals, nerve cells, and ganglion cells. It is specifically arranged around the nucleus, and is a type of lipochrome.
Formation and turnover
It appears to be the product of the oxidation of unsaturated fatty acids and may be symptomatic of membrane damage, or damage to mitochondria and lysosomes. Aside from a large lipid content, lipofuscin is known to contain sugars and metals, including mercury, aluminum, iron, copper and zinc.[2]
The accumulation of lipofuscin-like material may be the result of an imbalance between formation and disposal mechanisms: Such accumulation can be induced in rats by administering a protease inhibitor (leupeptin); after a period of three months, the levels of the lipofuscin-like material return to normal, indicating the action of a significant disposal mechanism.[3] However, this result is controversial, as it is questionable if the leupeptin-induced material is true lipofuscin.[4][5] There exists evidence that "true lipofuscin" is not degradable in vitro;[6][7][8] whether this holds in vivo over longer time periods is not clear.
Relation to diseases

Lipofuscin accumulation is a major risk factor implicated in macular degeneration, a degenerative disease of the eye,[9] as well as Stargardt disease, an inherited juvenile form of macular degeneration.
Abnormal accumulation of lipofuscin is associated with a group of diseases of neurodegenerative disorder type called lipofuscinoses, e.g., neuronal ceroid lipofuscinosis, also known as Batten disease, as well as some other names.
Pathological accumulation of lipofuscin is implicated in Alzheimer's disease, Parkinson's disease, amyotrophic lateral sclerosis, certain lysosomal diseases, acromegaly, denervation atrophy, lipid myopathy, chronic obstructive pulmonary disease,[10] and centronuclear myopathy. Accumulation of lipofuscin in the colon is the cause of the condition melanosis coli.
Possible therapies
Calorie restriction,[2] vitamin E,[2] and increased glutathione appear to reduce or halt the production of lipofuscin.
The nootropic drug piracetam appears to significantly reduce accumulation of lipofuscin in the brain tissue of rats.[11]
Other possible treatments:
Wet macular degeneration can be treated using Selective Photothermolysis where a pulsed unfocused laser predominantly heats and kills pigment- (i.e.: lipofuscin-) rich cells, leaving untouched healthy cells to multiply and fill in the gaps. The technique is also used as a skin treatment to remove tattoos, liverspots, and in general make skin appear younger. This ability to selectively target lipofuscin has opened up research opportunities in the field of Anti-aging medicine.
A tetrahydropyridoether can remove lipofuscin from retinal pigment epithelial cells.[15] This opens up a new therapy option for the treatment of dry age-related macular degeneration and Stargardt disease, for which there is currently no treatment. The drug has now been granted orphan drug designation for the treatment of Stargardt disease by the European Medicines Agency.
Other uses
Lipofuscin quantification is used for age determination in various crustaceans such as lobsters and spiny lobsters.[16][17] Since these animals lack bony parts, they cannot be aged in the same way as bony fish, in which annual increments in the ear-bones or otoliths are commonly used. Age determination of fish and shellfish is a fundamental step in generating basic biological data such as growth curves, and is needed for many stock assessment methods. Several studies have indicated that quantifying the amount of lipofuscin present in the eye-stalks of various crustaceans can give an index of their age. This method has not yet been widely applied in fisheries management mainly due to problems in relating lipofuscin levels in wild-caught animals with accumulation curves derived from aquarium-reared animals.
See also
References
- ↑ "lipofuscin" at Dorland's Medical Dictionary
- 1 2 3 Chris Gaugler, "Lipofuscin", Stanislaus Journal of Biochemical Reviews May 1997
- ↑ Katz, ML; Rice, LM; Gao, CL (1999). "Reversible accumulation of lipofuscin-like inclusions in the retinal pigment epithelium". Investigative Ophthalmology & Visual Science. 40: 175–181.
- ↑ Terman, Alexei; Brunk, Ulf T. (1999). "Is Lipofuscin Eliminated from Cells?". Investigative Ophthalmology and Visual Science. 40: 2463–2464.
- ↑ Davies, Sallyanne; Ellis, Steven (1999). "Lipofuscin Turnover". Investigative Ophthalmology and Visual Science. 40: 1887–1888.
- ↑ Terman, A, Brunk, UT (1998). "On the degradability and exocytosis of ceroid/lipofuscin in cultured rat cardiac myocytes". Mech Ageing Dev. 100 (2): 145–156. doi:10.1016/S0047-6374(97)00129-2. PMID 9541135.
- ↑ Terman, A; Brunk, UT (1998). "Ceroid/lipofuscin formation in cultured human fibroblasts: the role of oxidative stress and lysosomal proteolysis". Mech Ageing Dev. 104: 277–291. doi:10.1016/s0047-6374(98)00073-6. PMID 9818731.
- ↑ Elleder, M; Drahota, Z; Lisá, V; Mares, V; Mandys, V; Müller, J; Palmer, DN (1995). "Tissue culture loading test with storage granules from animal models of neuronal ceroid-lipofuscinosis (Batten disease): testing their lysosomal degradability by normal and Batten cells". Am J Med Genet. 57: 213–221. doi:10.1002/ajmg.1320570220. PMID 7668332.
- ↑ John Lacey, "Harvard Medical signs agreement with Merck to develop potential therapy for macular degeneration", 23-May-2006
- ↑ Joakim Allaire, François Maltais, Pierre LeBlanc, Pierre-Michel Simard, François Whittom, Jean-François Doyon, Clermont Simard & Jean Jobin (2002). "Lipofuscin accumulation in the vastus lateralis muscle in patients with chronic obstructive pulmonary disease". Muscle and Nerve. 25 (3): 383–389. doi:10.1002/mus.10039.
- ↑ Paula-Barbosa, M.; et al. (1991). "The effects of Piracetam on lipofuscin of the rat cerebellar and hippocampa; neurons after long-term alcohol treatment and withdrawal". Alcoholism: Clinical and Experimental Research. 15: 834–838. doi:10.1111/j.1530-0277.1991.tb00610.x.
- ↑ Roy, D; Pathak, DN; Singh, R (1983). "Effect of centrophenoxine on the antioxidative enzymes in various regions of the aging rat brain.". Exp Gerontol. 18 (3): 185–97. doi:10.1016/0531-5565(83)90031-1. PMID 6416880.
- ↑ Amenta F, Ferrante F, et al., Reduced lipofuscin accumulation in senescent rat brain by long-term acetyl-L-carnitine treatment. Arch Gerontol Geriatr. 1989 Sep-Oct;9(2):147-53.
- ↑ Huang, SZ; Luo, YJ; Wang, L; Cai, KY (Jan 2005). "Effect of ginkgo biloba extract on livers in aged rats.". World J Gastroenterol. 11 (1): 132–5. doi:10.3748/wjg.v11.i1.132. PMID 15609412.
- ↑ Julien, S; Schraermeyer, U (Oct 2012). "Lipofuscin can be removed from the retinal pigment epithelium of monkeys". Neurobiol Aging. 33 (10): 2390–7. doi:10.1016/j.neurobiolaging.2011.12.009.
- ↑ Ingebrigt Uglem, Mark Belchier & Terje Svåsand (2005). "Age determination of European lobsters (Homarus gammarus L.) by histological quantification of lipofuscin". Journal of Crustacean Biology. 25 (1): 95–99. doi:10.1651/c-2448. JSTOR 1549930.
- ↑ Kerry E. Maxwell, Thomas R. Matthews, Matt R. J. Sheehy, Rodney D. Bertelsen & Charles D. Derby (2007). "Neurolipofuscin is a measure of age in Panulirus argus, the Caribbean spiny lobster, in Florida". The Biological Bulletin. 213 (1): 55–66. JSTOR 25066618.
External links for general reviews
- Terman A, Brunk U (2004). "Lipofuscin". Int J Biochem Cell Biol. 36 (8): 1400–4. doi:10.1016/j.biocel.2003.08.009. PMID 15147719.
- Histology at neuro.wustl.edu
- Histology image: 20301loa – Histology Learning System at Boston University
- Destroying Lipofuscin and Destroying Cancer, FightAging.org
- Unfocused Pulsed Lasers Selectively Destroy Lipofuscin, AcceleratingFuture.com