Lanthanum oxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Lanthanum(III) oxide | |
| Other names
Lanthanum sesquioxide Lanthana | |
| Identifiers | |
| 1312-81-8 | |
| ChemSpider | 2529886 133008 |
| ECHA InfoCard | 100.013.819 |
| PubChem | 150906 |
| RTECS number | OE5330000 |
| |
| Properties | |
| La2O3 | |
| Molar mass | 325.809 g/mol |
| Appearance | White powder, hygroscopic |
| Density | 6.51 g/cm3, solid |
| Melting point | 2,315 °C (4,199 °F; 2,588 K) |
| Boiling point | 4,200 °C (7,590 °F; 4,470 K) |
| Insoluble | |
| Band gap | 4.3 eV |
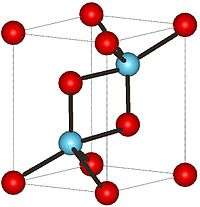
| Structure | |
| Hexagonal, hP5 | |
| P-3m1, No. 164 | |
| Hazards | |
| Main hazards | Irritant |
| R-phrases | R36/37 |
| S-phrases | S26, S22, S37/39 |
| NFPA 704 | |
| Flash point | Non-flammable |
| Related compounds | |
| Other anions |
Lanthanum(III) chloride |
| Other cations |
Cerium(III) oxide Scandium(III) oxide Yttrium(III) oxide Actinium(III) oxide |
| Related compounds |
Lanthanum aluminium oxide, LaSrCoO4 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Lanthanum oxide is La2O3, an inorganic compound containing the rare earth element lanthanum and oxygen. It is used to develop ferroelectric materials, as a component of optical materials, and is a feedstock for certain catalysts.
Properties

La2O3 has a band gap at approximately 5.8 eV.[1] It has the lowest lattice energy, with very high dielectric constant, ε = 27. La2O3 is widely used in industry as well as in the research laboratory. Lanthanum oxide is an odorless, white solid that is insoluble in water, but soluble in dilute acid. Depending on the pH of the compound, different crystal structures can be obtained. La2O3 is hygroscopic; under atmosphere, lanthanum oxide absorbs moisture over time and converts to lanthanum hydroxide. Lanthanum oxide has p-type semiconducting properties. Its resistivity decreases with an increase in temperature. Its average room temperature resistivity is 10 kΩ·cm.
Structure
At low temperatures, La2O3 has an A-M2O3 hexagonal crystal structure. The La3+ metal atoms are surrounded by a 7 coordinate group of O2−atoms, the oxygen ions are in an octahedral shape around the metal atom and there is one oxygen ion above one of the octahedral faces.[2] On the other hand, at high temperatures the Lanthanum oxide converts to a C-M2O3 cubic crystal structure. The La3+ ion is surrounded by a 6 coordinate group of O2− ions.[3]
Elements obtained from Lanthana
There are several elements which were discovered as a consequence of the lengthy analysis and breakdown of the ore Gadolinite. As the ore was progressively analysed further, the residue was given the label thence ceria and thence lanthana and onwards to yttria, erbia, and terbia. The list by date includes Cerium 58, Lanthanum 57, Erbium 68, Terbium 65, Yttrium 39, Ytterbium 70, Holmium 67, Thulium 69, Scandium 21, Praseodymium 59, Neodymium 60 and Dysprosium 66. Several of these new elements were either discovered or isolated by Carl Gustaf Mosander in the 1830s and 1840s.
Synthesis
Lanthanum oxide crystallizes as several polymorphs.
To produce hexagonal La2O3, a 0.1 M solution of LaCl3 is sprayed onto a preheated substrate, usually made of metal chalcogenides.[4] The process can be viewed as occurring in two steps – hydrolysis followed by dehydration:
- 2 LaCl3 + 3 H2O → La(OH)3 + 3 HCl
- 2 La(OH)3 → La2O3 + 3 H2O
An alternative route to hexagonal La2O3 involves precipitation of nominal La(OH)3 from aqueous solution using a combination of 2.5% NH3 and the surfactant sodium dodecyl sulfate followed by heating and stirring for 24 hours at 80 °C:
- 2 LaCl3+ 3 H2O + 3 NH3 → La(OH)3 + 3 NH4Cl
- LaCl3·3H2O → La2O3
Other routes include:
- 2 La2S3 + 3 CO2 → 2 La2O3 + 3 CS2
- 2 La2(SO4)3 + heat → 2 La2O3 + 6 SO3
Reactions
Lanthanum oxide is used to develop ferroelectric materials, such as La-doped Bi4Ti3O12 (BLT). Lanthanum oxide is used in optical materials, often the optical glasses are doped with La2O3 to improve the glass' refractive index, chemical durability, and mechanical strength.
- 3 B2O3 + La2O3 → 2 La(BO2)3
When this 1:3 reaction is mixed into a glass composite, the high molecular weight of the lanthanum causes an increase of the homogeneous mixture of the melt which leads to a lower melting point.[5] The addition of the La2O3 to the glass melt leads to a higher glass transition temperature from 658 °C to 679 °C. The addition also leads to a higher density, microhardness, and refractive index of the glass.
Uses and applications
La2O3 is used to make optical glasses, to which this oxide confers increased density, refractive index, and hardness. Together with oxides of tungsten, tantalum, and thorium, La2O3 improves the resistance of the glass to attack by alkali. La2O3 is an ingredient for the manufacture of piezoelectric and thermoelectric materials. Automobile exhaust-gas converters contain La2O3.[6] La2O3 is also used in X-ray imaging intensifying screens, phosphors as well as dielectric and conductive ceramics. Gives of bright glow.
La2O3 has been examined for the oxidative coupling of methane.[7]
La2O3 films can be deposited by many different methods, including: chemical vapor disposition, atomic layer deposition, thermal oxidation, sputtering, and spray pyrolysis. Depositions of these films occur in a temperature range of 250–450 °C. Polycrystalline films are formed at 350 °C.[4]
La2O3 tungsten electrodes are replacing thorianated tungsten electrodes in Gas tungsten arc welding (TIG) due to safety concerns with thorium's radioactivity.
References
- ↑ Shang, G.; Peacock, P. W.; Robertson, J. "Stability and band offsets of nitrogenated high-dielectric-constant gate oxides". Applied Physics Letters. 84: 106–108. doi:10.1063/1.1638896.
- ↑ Wells, A.F. (1984). Structural Inorganic Chemistry. Oxford: Clarendon Press. p. 546.
- ↑ Wyckoff, R. W.G. (1963). Crystal Structures: Inorganic Compounds RXn, RnMX2, RnMX3. New York: Interscience Publishers.
- 1 2 Kale, S.S.; Jadhav, K.R.; Patil, P.S.; Gujar, T.P.; Lokhande, C.D. (2005). "Characterizations of spray-deposited lanthanum oxide (La2O3) thin films". Materials Letters. 59: 3007–3009. doi:10.1016/j.matlet.2005.02.091.
- ↑ Vinogradova, N. N.; Dmitruk, L. N.; Petrova, O. B. (2004). "Glass Transition and Crystallization of Glasses Based on Rare-Earth Borates". Glass Physics and Chemistry. 30: 1–5. doi:10.1023/B:GPAC.0000016391.83527.44.
- ↑ Cao, J; Ji, H; Liu, J; Zheng, M; Chang, X; Ma, X; Zhang, A; Xu, Q (2005). "Controllable syntheses of hexagonal and lamellar mesostructured lanthanum oxide". Materials Letters. 59: 408–411. doi:10.1016/j.matlet.2004.09.034.
- ↑ O.V. Manoilova; et al. (2004). "Surface Acidity and Basicity of La2O3, LaOCl, and LaCl3 Characterized by IR Spectroscopy, TPD, and DFT Calculations". J. Phys. Chem. B. 108: 15770–15781. doi:10.1021/jp040311m.
External links
- http://www.rsc.org/periodic-table/element/57/lanthanum
- External MSDS 1
- External MSDS 2
- Lanthanum Oxide MSDS
