Lamina propria
| Lamina propria | |
|---|---|
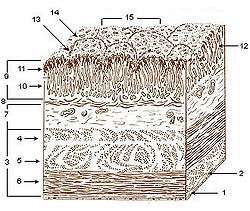 Layers of stomach wall: 1. Serosa 2. Tela subserosa 3. Muscularis 4. Oblique fibers of muscle wall 5. Circular muscle layer 6. Longitudinal muscle layer 7. Submucosa 8. Lamina muscularis mucosae 9. Mucosa 10. Lamina propria 11. Epithelium 12. Gastric glands 13. Gastric pits 14. Villous folds 15. Gastric areas (gastric surface) | |
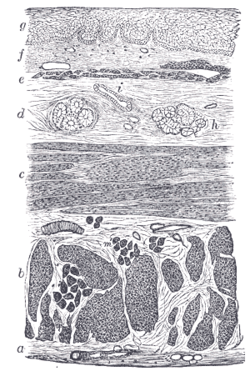 Section of the human esophagus. Moderately magnified. The section is transverse and from near the middle of the gullet. a. Fibrous covering. b. Divided fibers of longitudinal muscular coat. c. Transverse muscular fibers. d. Submucous or areolar layer. e. Muscularis mucosae. f. Mucous membrane, with vessels and part of a lymphoid nodule. g. Stratified squamous epithelium. h. Mucous gland. i. Gland duct. m. Striated muscular fibers cut across. | |
| Identifiers | |
| FMA | 62517 |
The lamina propria is a constituent of the moist linings known as mucous membranes or mucosa, which line various tubes in the body (such as the respiratory tract, the gastrointestinal tract, and the urogenital tract).
The lamina propria (more correctly lamina propria mucosæ) is a thin layer of loose connective tissue, or dense irregular connective tissue, which lies beneath the epithelium and together with the epithelium constitutes the mucosa. As its Latin name indicates, it is a characteristic component of the mucosa, "the mucosa's own special layer". Thus the term mucosa or mucous membrane always refers to the combination of the epithelium plus the lamina propria.[1]
The connective tissue of the lamina propria is very loose, allowing it to be very cell rich. The cell population of the lamina propria is variable and can include, for example, fibroblasts, lymphocytes, plasma cells, macrophages, eosinophilic leukocytes, and mast cells.[2] It provides support and nutrition to the epithelium, as well as the means to bind to the underlying tissue. Irregularities in the connective tissue surface, such as papillae found in the tongue, increase the area of contact of the lamina propria and the epithelium.[3]
The lamina propria contains capillaries and a central lacteal (lymph vessel) in the small intestine, as well as lymphoid tissue. Lamina propria also contains glands with the ducts opening on to the mucosal epithelium, that secrete mucus and serous secretions. The lamina propria is also rich in immune cells known as lymphocytes. A majority of these cells are IgA-secreting B cells.
Composition and importance
The lamina propria is a loose connective tissue, hence it is not as fibrous as the underlying connective tissue of the submucosa.[4] The connective tissue and architecture of the lamina propria is very compressible and elastic, this can be seen in organs that require expansion such as the bladder.[5] The collagen in the lamina propria of elastic organs has been shown to play a major role in mechanical function. In the bladder the collagen composition of its lamina propria allows for structure, tensile strength, and compliance, through complex coiling.[6] It has been suggested that myofibroblasts also reside in the lamina propria of several organs. These cells have characteristics of both smooth muscle and fibroblasts.[7]
The lamina propria may also be rich in vascular networks, lymphatic vessels, elastic fibers, and smooth muscle fascicles from the muscularis mucosae. Afferent and efferent nerve endings can be found in the lamina propria as well.[6] Immune cells as well as lymphoid tissue, including lymphoid nodules and capillaries, may be present. Smooth muscle fibers may be in the lamina propria of some tissues, such as the intestinal villi. It is practically void of fat cells.[4] Lymphatics penetrate the mucosa and lie below the basement membrane of the epithelium, from there they drain the lamina propria.[8] The fast rate of cell death and regeneration of the epithelium leaves behind many apoptotic cell bodies. These have been found to go into the lamina propria, most of which are inside its macrophages.[9]
Role in the immune system
Because the epithelium is often under external stress and is somewhat delicate, the lamina propria hosts many immune cells.[4] In the intestinal tract the immune system must have tolerance to the normal intestinal flora, yet respond to pathogenic microorganisms. Imbalance of this causes inflammation diseases such as inflammatory bowel disease.[10] The lamina propria’s richness in macrophages and lymphoid cells makes it a key place for immune responses to occur. It forms part of the barrier that protects internal tissues from external pathogenic microorganisms, especially from the gastrointestinal tract.[11]
The myofibroblasts in the lamina propria make it a very important contributor of inflammation and wound healing responses. Myofibroblasts are capable of releasing cytokines and chemokines in response to stress. Also their contractile capacity may help pull tissue together in the wound healing mechanism.[7]
Cancer
Progression of epithelial cancer often relies on deep and regional lymph node invasion [12] The lamina propria being one of the barriers to the submucosa is an area where epithelial cancer invasion is of significance since lymphatic invasion is an independent predictor of lymph node metastasis, especially in gastric cancer.[13] As soon as the tumors breach the basement membrane and reach the lamina propria, they are exposed to lymphatics which may increase the rate of metastasis and cancer progression. Deeper invasion into the submucosa will increase the exposure to lymphatics.[8]
Long-standing inflammation is a risk factor for the development of cancer. The lamina propria macrophages when under much stress release pro-inflammatory signals that may lead to increased probability of developing cancer. An example of this is the over activation of the IL-6/STAT3 pathway, which has been linked to colitis-associated cancer.[14]
See also
- Submucosa
- Basal lamina (part of which is also known as Lamina densa)
- Myofibroblast
References
- ↑ Burkitt, H. George; Young, Barbara; Heath, John W., eds. (1993). Wheater's Functional Histology (3rd ed.). ISBN 978-0-443-04691-9.
- ↑ Slomianka, Lutz (2009). "Blue Histology-Gastrointestinal Tract". The University of Western Australia.
- ↑ Mescher, Anthony (2009). Junqueira's Basic Histology: Text & Atlas (12th ed.). ISBN 978-0-07-171475-4.
- 1 2 3 King, David (2009). "Study Guide: Histology of the Gastrointestinal System".
- ↑ Chang, S. L.; Chung, J. S.; Yeung, M. K.; Howard, P. S.; MacArak, E. J. (1999). "Roles of the Lamina Propria and the Detrusor in Tension Transfer during Bladder Filling". Scandinavian Journal of Urology and Nephrology. 33: 38–45. doi:10.1080/003655999750042132. PMID 10573775.
- 1 2 Andersson, Karl-Erik; McCloskey, Karen D. (2014). "Lamina propria: The functional center of the bladder?". Neurourology and Urodynamics. 33 (1): 9–16. doi:10.1002/nau.22465. PMID 23847015.
- 1 2 Wiseman, O.J.; Fowler, C.J.; Landon, D.N. (2003). "The role of the human bladder lamina propria myofibroblast". BJU International. 91 (1): 89–93. doi:10.1046/j.1464-410X.2003.03802.x. PMID 12614258.
- 1 2 Rice, Thomas W; Zuccaro, Gregory; Adelstein, David J; Rybicki, Lisa A; Blackstone, Eugene H; Goldblum, John R (1998). "Esophageal Carcinoma: Depth of Tumor Invasion is Predictive of Regional Lymph Node Status". The Annals of Thoracic Surgery. 65 (3): 787–92. doi:10.1016/S0003-4975(97)01387-8. PMID 9527214.
- ↑ Hall, Peter A.; Coates, Philip J.; Ansari, Bijan; Hopwood, David (1994). "Regulation of cell number in the mammalian gastrointestinal tract: The importance of apoptosis". Journal of Cell Science. 107 (12): 3569–77. PMID 7706406.
- ↑ Varol, Chen; Vallon-Eberhard, Alexandra; Elinav, Eran; Aychek, Tegest; Shapira, Yami; Luche, Hervé; Fehling, Hans Jörg; Hardt, Wolf-Dietrich; Shakhar, Guy; Jung, Steffen (2009). "Intestinal Lamina Propria Dendritic Cell Subsets Have Different Origin and Functions". Immunity. 31 (3): 502–12. doi:10.1016/j.immuni.2009.06.025. PMID 19733097.
- ↑ Bischoff, Stephan C; Barbara, Giovanni; Buurman, Wim; Ockhuizen, Theo; Schulzke, Jörg-Dieter; Serino, Matteo; Tilg, Herbert; Watson, Alastair; Wells, Jerry M (2014-11-18). "Intestinal permeability – a new target for disease prevention and therapy". BMC Gastroenterology. 14. doi:10.1186/s12876-014-0189-7. ISSN 1471-230X. PMC 4253991
 . PMID 25407511.
. PMID 25407511. - ↑ Lee, Ji Yong; Joo, Hee Jae; Cho, Dae Sung; Kim, Sun Il; Ahn, Hyun Soo; Kim, Se Joong (2012). "Prognostic Significance of Substaging according to the Depth of Lamina Propria Invasion in Primary T1 Transitional Cell Carcinoma of the Bladder". Korean Journal of Urology. 53 (5): 317–23. doi:10.4111/kju.2012.53.5.317. PMC 3364470
 . PMID 22670190.
. PMID 22670190. - ↑ Yonemura, Yutaka; Endou, Yoshio; Tabachi, Kayoko; Kawamura, Taiichi; Yun, Hyo-Yung; Kameya, Toru; Hayashi, Isamu; Bandou, Etsurou; Sasaki, Takuma; Miura, Masahiro (2006). "Evaluation of lymphatic invasion in primary gastric cancer by a new monoclonal antibody, D2-40". Human Pathology. 37 (9): 1193–9. doi:10.1016/j.humpath.2006.04.014. PMID 16938525.
- ↑ Matsumoto, Satoshi; Hara, Taeko; Mitsuyama, Keiichi; Yamamoto, Mayuko; Tsuruta, Osamu; Sata, Michio; Scheller, Jürgen; Rose-John, Stefan; Kado, Sho-ichi; Takada, Toshihiko (2009). "Essential Roles of IL-6 Trans-Signaling in Colonic Epithelial Cells, Induced by the IL-6/Soluble-IL-6 Receptor Derived from Lamina Propria Macrophages, on the Development of Colitis-Associated Premalignant Cancer in a Murine Model". The Journal of Immunology. 184 (3): 1543–51. doi:10.4049/jimmunol.0801217. PMID 20042582.
External links
- Anatomy Atlases - Microscopic Anatomy, plate 10.198
- Histology image: 10802loa – Histology Learning System at Boston University - "Digestive System: Alimentary Canal - esophagus "
- Histology image: 03301loa – Histology Learning System at Boston University - "Connective Tissue: lamina propria; loose connective tissue "
- UIUC Histology Subject 272
- Anatomy photo: Digestive/mammal/system1/system3 - Comparative Organology at University of California, Davis - "Mammal, whole system (LM, Low)"
- Slide at ucla.edu