Laboratory mouse

The laboratory mouse is a small mammal of the order Rodentia which is bred and used for scientific research. Laboratory mice are usually of the species Mus musculus. They are the most commonly used mammalian research model and are used for research in genetics, psychology, medicine and other scientific disciplines. Mice belong to the Euarchontoglires clade, which includes humans. This close relationship, the associated high homology with humans, their ease of maintenance and handling, and their high reproduction rate, make mice particularly suitable models for human-oriented research. The laboratory mouse genome has been sequenced and many mouse genes have human homologues.[1]
Other mouse species sometimes used in laboratory research include the American white-footed mouse (Peromyscus leucopus) and the deer mouse (Peromyscus maniculatus).
History as a biological model
Mice have been used in biomedical research since the 16th Century when William Harvey used them for his studies on reproduction and blood circulation and Robert Hooke used them to investigate the biological consequences of an increase in air pressure.[2] During the 18th Century Joseph Priestley and Antoine Lavoisier both used mice to study respiration. In the 19th Century Gregor Mendel carried out his early investigations of inheritance on mouse coat color but was asked by his superior to stop breeding in his cell "smelly creatures that, in addition, copulated and had sex".[2] He then switched his investigations to peas but, as his observations were published in a somewhat obscure botanical journal, they were virtually ignored for over 35 years until they were rediscovered in the early 20th Century. In 1902 Lucien Cuénot published the results of his experiments using mice which showed that Mendel's laws of inheritance were also valid for animals — results that were soon confirmed and extended to other species.[2]
In the early part of the 20th century Clarence Cook Little, a Harvard undergraduate was conducting studies on mouse genetics in the laboratory of William Ernest Castle. Little and Castle collaborated closely with Abbie Lathrop who was a breeder of fancy mice and rats which she marketed to rodent hobbyists and keepers of exotic pets, and later began selling in large numbers to scientific researchers.[3] Together they generated the DBA (Dilute, Brown and non-Agouti) inbred mouse strain and initiated the systematic generation of inbred strains.[4] The mouse has since been used extensively as a model organism and is associated with many important biological discoveries of the 20th and 21st Centuries.[2]
Reproduction
Breeding onset occurs at about 50 days of age in both females and males, although females may have their first estrus at 25–40 days. Mice are polyestrous and breed year round; ovulation is spontaneous. The duration of the estrous cycle is 4–5 days and lasts about 12 hours, occurring in the evening. Vaginal smears are useful in timed matings to determine the stage of the estrous cycle. Mating can be confirmed by the presence of a copulatory plug in the vagina up to 24 hours post-copulation. The presence of sperm on a vaginal smear is also a reliable indicator of mating.[5]
The average gestation period is 20 days. A fertile postpartum estrus occurs 14–24 hours following parturition, and simultaneous lactation and gestation prolongs gestation by 3–10 days owing to delayed implantation. The average litter size is 10–12 during optimum production, but is highly strain-dependent. As a general rule, inbred mice tend to have longer gestation periods and smaller litters than outbred and hybrid mice. The young are called pups and weigh 0.5–1.5 g (0.018–0.053 oz) at birth, are hairless, and have closed eyelids and ears. Pups are weaned at 3 weeks of age when they weigh about 10–12 g (0.35–0.42 oz). If the female does not mate during the postpartum estrus, she resumes cycling 2–5 days post-weaning.[5]
Newborn males are distinguished from newborn females by noting the greater anogenital distance and larger genital papilla in the male. This is best accomplished by lifting the tails of littermates and comparing perineums.[5]
Genetics and strains
Mice are mammals of the Glires clade ( a group consisting of an ancestor and all its descendants), which means they are amongst the closest relatives of humans along with lagomorphs, treeshrews, flying lemurs and other primates.
| Euarchontoglires |
| |||||||||||||||||||||||||||
| |
Laboratory mice are the same species as the house mouse, however, they are often very different in behaviour and physiology. There are hundreds of established inbred, outbred, and transgenic strains. A strain, in reference to rodents, is a group in which all members are as nearly as possible genetically identical. In laboratory mice, this is accomplished through inbreeding. By having this type of population, it is possible to conduct experiments on the roles of genes, or conduct experiments that exclude genetic variation as a factor. In contrast, outbred populations are used when identical genotypes are unnecessary or a population with genetic variation is required, and are usually referred to as stocks rather than strains.[6][7] Over 400 standardized, inbred strains have been developed.
Most laboratory mice are hybrids of different subspecies, most commonly of Mus musculus domesticus and Mus musculus musculus. Laboratory mice can have a variety of coat colours, including agouti, black and albino. Many (but not all) laboratory strains are inbred. The different strains are identified with specific letter-digit combinations; for example C57BL/6 and BALB/c. The first such inbred strains were produced in 1909 by Clarence Cook Little, who was influential in promoting the mouse as a laboratory organism.[8] In 2011, an estimated 83% of laboratory rodents supplied in the U.S. were C57BL/6 laboratory mice.[9]
Genome
Sequencing of the laboratory mouse genome was completed in late 2002 using the C57BL/6 strain. This was only the second mammalian genome to be sequenced after humans.[9] The haploid genome is about three billion base pairs long (3,000 Mb distributed over 20 chromosomes), therefore equal to the size of the human genome. Estimating the number of genes contained in the mouse genome is difficult, in part because the definition of a gene is still being debated and extended. The current count of primary coding genes in the laboratory mouse is 23,139.[10] compared to an estimated 20,774 in humans.[11]
Mutant and transgenic strains

Various mutant strains of mice have been created by a number of methods. A small selection from the many available strains includes -
- Mice resulting from ordinary breeding
- Non-obese diabetic (NOD) mice, which develop diabetes mellitus type 1.
- Murphy Roths Large (MRL) mice with unusual regenerative capacities[12]
- "Waltzing" mice, which walk in a circular pattern due to a mutation adversely affecting their inner ears
- Immunodeficient nude mice, lacking hair and a thymus: The mice do not produce T lymphocytes, therefore do not mount cellular immune responses. They are used for research in immunology and transplantation
- Severe combined immunodeficient, with an almost completely defective immune system
- Transgenic mice, with foreign genes inserted into their genome
- Abnormally large mice, with an inserted rat growth hormone gene
- Oncomice, with an activated oncogene, so as to significantly increase the incidence of cancer
- Doogie mice, with enhanced NMDA receptor function, resulting in improved memory and learning
- Knockout mice, where a specific gene was made inoperable by a technique known as gene knockout: The purpose is to study the function of the gene's product or to simulate a human disease.
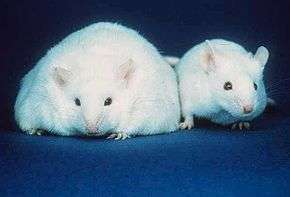
- Fat mice, prone to obesity due to a carboxypeptidase E deficiency
- Strong muscular mice, with a disabled myostatin gene, nicknamed "mighty mice."
Since 1998, it has been possible to clone mice from cells derived from adult animals.
Appearance and behaviour
Laboratory mice have retained many of the physical and behavioural characteristics of house mice, however, due to many generations of artificial selection some of these characteristics now vary markedly. Due to the large number of strains of laboratory mice, it is impractical to comprehensively describe the appearance and behaviour of all these, however, they are described below for two of the most commonly used strains.
C57BL/6

C57BL/6 mice have a dark brown, nearly black coat. They are more sensitive to noise and odours and are more likely to bite than the more docile laboratory strains such as BALB/c.[13]
Group-housed C57BL/6 mice (and other strains) display barbering behaviour, in which the dominant mouse in a cage selectively removes hair from its subordinate cage mates.[14] Mice that have been barbered extensively can have large bald patches on their bodies, commonly around the head, snout, and shoulders, although barbering may appear anywhere on the body. Both hair and vibrissae may be removed. Barbering is more frequently seen in female mice; male mice are more likely to display dominance through fighting.[15]
C57BL/6 has several unusual characteristics which make it useful for some research studies but inappropriate for others: It is unusually sensitive to pain and to cold, and analgesic medications are less effective in this strain.[16] Unlike most laboratory mouse strains, the C57BL/6 drinks alcoholic beverages voluntarily. It is more susceptible than average to morphine addiction, atherosclerosis, and age-related hearing loss.[9]
BALB/c

BALB/c is an albino, laboratory-bred strain from which a number of common substrains are derived. With over 200 generations bred since 1920, BALB/c mice are distributed globally and are among the most widely used inbred strains used in animal experimentation.[17]
BALB/c are noted for displaying high levels of anxiety and for being relatively resistant to diet-induced atherosclerosis, making them a useful model for cardiovascular research.[18][19]
Male BALB/c mice are aggressive and will fight other males if housed together. However, the BALB/Lac substrain is much more docile.[20] Most BALB/c mice substrains have a long reproductive life-span.[17]
There are noted differences between different BALB/c substrains, though these are thought to be due to mutation rather than genetic contamination.[21] The BALB/cWt is unusual in that 3% of progeny display true hermaphroditism.[22]
Husbandry

Nutrition
In nature, mice are usually herbivores, consuming a wide range of fruit or grain.[23] However, in laboratory studies it is usually necessary to avoid biological variation and to achieve this, laboratory mice are almost always fed only commercial pelleted mouse feed. Food intake is approximately 15 g (0.53 oz) per 100 g (3.5 oz) of body weight per day; water intake is approximately 15 ml (0.53 imp fl oz; 0.51 US fl oz) per 100 g of body weight per day.[5]
Injection procedures
Routes of administration of injections in laboratory mice are mainly subcutaneous, intraperitoneal and intravenous. Intramuscular administration is not recommended due to small muscle mass.[24] Intracerebral administration is also possible. Each route has a recommended injection site, approximate needle gauge and recommended maximum injected volume at a single time at one site, as given in the table below:
| Route | Recommended site[24] | Needle gauge[24] | Maximal volume[25] |
|---|---|---|---|
| subcutaneous | dorsum, between scapula | 25-26 ga | 2-3 ml |
| intraperitoneal | left lower quadrant | 25-27 ga | 2-3 ml |
| intravenous | lateral tail vein | 27-28 ga | 0.2 ml |
| intramuscular | hindlimb, caudal thigh | 26-27 ga | 0.05 ml |
| intracerebral | cranium | 27 ga |
To facilitate intravenous injection into the tail, laboratory mice can be carefully warmed under heat lamps to vasodilate the vessels.[24]
Anaesthesia
A common regimen for general anesthesia for the house mouse is ketamine (in the dose of 100 mg per kg body weight) plus xylazine (in the dose of 5–10 mg per kg), injected by the intraperitoneal route.[26] It has a duration of effect of about 30 minutes.[26]
Euthanasia
Approved procedures for euthanasia of laboratory mice include compressed CO2 gas, injectable barbiturate anesthetics, inhalable anesthetics, such as Halothane, and physical methods, such as cervical dislocation and decapitation.[27] In 2013, the American Veterinary Medical Association issued new guidelines for CO2 induction, stating that a flow rate of 10% to 30% volume/min is optimal for euthanasing laboratory mice.[28]
Pathogen susceptibility
A recent study detected a murine astrovirus in laboratory mice held at more than half of the US and Japanese institutes investigated.[29] Murine astrovirus was found in nine mice strains, including NSG, NOD-SCID, NSG-3GS, C57BL6-Timp-3−/−, uPA-NOG, B6J, ICR, Bash2, and BALB/C, with various degrees of prevalence. The pathogenicity of the murine astrovirus was not known.
Legislation in research
United Kingdom
In the UK, as with all other vertebrates and some invertebrates, any scientific procedure which is likely to cause "pain, suffering, distress or lasting harm" is regulated by the Home Office under the Animals (Scientific Procedures) Act 1986. UK regulations are considered amongst the most comprehensive and rigorous in the world.[30] Detailed data on the use of laboratory mice (and other species) in research in the UK are published each year.[31] In the UK in 2013, there were a total of 3,077,115 regulated procedures on mice in scientific procedure establishments, licensed under the Act.[32]
United States
In the US, laboratory mice are not regulated under the Animal Welfare Act administered by the USDA APHIS. However, the Public Health Service Act (PHS) as administered by the National Institutes of Health does offer a standard for their care and use. Compliance with the PHS is required for a research project to receive federal funding. PHS policy is administered by the Office of Laboratory Animal Welfare. Many academic research institutes seek accreditation voluntarily, often through the Association for Assessment and Accreditation of Laboratory Animal Care, which maintains the standards of care found within The Guide for the Care and Use of Laboratory Animals and the PHS policy. This accreditation is voluntary, not a prerequisite, for federal funding.[33]
Limitations
While mice are by far the most widely used animals in biomedical research, recent studies have highlighted their limitations.[34] For example, the utility of rodents in testing for sepsis,[35] burns,[35] inflammation,[35] stroke,[36][37] ALS,[38][39][40] Alzheimer’s,[41] diabetes,[42][43] cancer,[44][45][46][47][48] multiple sclerosis,[49] Parkinson’s disease[49] and other illnesses has been called into question by a number of researchers. Regarding experiments on mice, some researchers have complained that “years and billions of dollars have been wasted following false leads” as a result of a preoccupation with the use of these animals in studies.[34]
An article in The Scientist notes, “The difficulties associated with using animal models for human disease result from the metabolic, anatomic, and cellular differences between humans and other creatures, but the problems go even deeper than that” including issues with the design and execution of the tests themselves.[37]
For example, researchers have found that many mice in laboratories are obese from excess food and minimal exercise which alters their physiology and drug metabolism.[50] Many laboratory animals, including mice, are chronically stressed which can also negatively affect research outcomes and the ability to accurately extrapolate findings to humans.[51][52] Researchers have also noted that many studies involving mice are poorly designed, leading to questionable findings.[37][39][40]
See also
- Laboratory rat
- Animal testing
- Animal testing on rodents
- Animal model
- Animal Identification
- Mouse models of colorectal and intestinal cancer
- Testing cosmetics on animals
References
- ↑ "MGI — Biology of the Laboratory Mouse". Informatics.jax.org. Retrieved 2010-07-29.
- 1 2 3 4 Hedrich, Hans (ed.). "The house mouse as a laboratory model: a historical perspective". The Laboratory Mouse. Elsevier Science. ISBN 9780080542539.
- ↑ Steensma, David P.; Kyle Robert A.; Shampo Marc A. (November 2010). "Abbie Lathrop, the "Mouse Woman of Granby": Rodent Fancier and Accidental Genetics Pioneer". Mayo Clinic Proceedings. Mayo Foundation for Medical Education and Research. 85 (11): e83. doi:10.4065/mcp.2010.0647. PMC 2966381
 . PMID 21061734.
. PMID 21061734. - ↑ Pillai, Shiv. "History of Immunology at Harvard". Harvard Medical School:About us. Harvard Medical School. Retrieved 19 December 2013.
- 1 2 3 4 Louisiana Veterinary Medical Association
- ↑ "Rules and guidelines for nomenclature of mouse and rat strains".
- ↑ "Outbred stocks".
- ↑ Crow JF (August 2002). "C. C. Little, cancer and inbred mice". Genetics. 161 (4): 1357–61. PMC 1462216
 . PMID 12196385.
. PMID 12196385. - 1 2 3 Engber, D. (2011). "The trouble with Black-6". Retrieved November 19, 2013.
- ↑ "Mouse assembly and gene annotation". Ensembl. Retrieved 29 July 2013.
- ↑ "Human assembly and gene annotation". Ensembl. Retrieved 29 July 2013.
- ↑ "JAX Mice Database — 002983 MRL.CBAJms-Fas/J". Jaxmice.jax.org. Retrieved 2010-07-29.
- ↑ Connor, A..B. (2006). "Aurora's Guide to Mo use Colony Management" (PDF). Cell Migration Gateway. CMC Activity Center. Retrieved 19 December 2013.
- ↑ Garner, J.P.; Weisker, S.M.; Dufour, B.; Mench, J.A. (2004). "Barbering (fur and whisker trimming) by laboratory mice as a model of human trichotillomania and obsessive-compulsive spectrum disorders" (PDF). Comparative Medicine. 54: 216–24.
- ↑ Sarna JR, Dyck RH, Whishaw IQ (February 2000). "The Dalila effect: C57BL6 mice barber whiskers by plucking". Behavioural Brain Research. 108 (1): 39–45. doi:10.1016/S0166-4328(99)00137-0. PMID 10680755. Retrieved 2011-01-16.
- ↑ Mogil JS, Wilson SG, Bon K, et al. (March 1999). "Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception". Pain. 80 (1-2): 67–82. doi:10.1016/s0304-3959(98)00197-3. PMID 10204719.
- 1 2 "BALB/c". Inbred Strains of Mice. Jackson Laboratory. Retrieved 2007-04-16.
- ↑ "BALB/cByJ". Jax Mice Data Sheet. Jackson Laboratory. Archived from the original on November 16, 2006. Retrieved 2007-04-16.
- ↑ "BALB/cJ". Jax Mice Data Sheet. Jackson Laboratory. Archived from the original on 11 April 2007. Retrieved 2007-04-16.
- ↑ Southwick, C. H.; Clark, L. H. (1966). "Aggressive behaviour and exploratory activity in fourteen mouse strains". Am. Zool. 6: 559.
- ↑ Hilgers, J.; van Nie, R.; Ivanyi, D.; Hilkens, J.; Michalides, R.; de Moes, J.; Poort-Keesom, R.; Kroezen, V.; von Deimling, O.; Kominami, R.; Holmes, R. (1985). "Genetic differences in BALB/c sublines". Curr. Top. Microbiol. Immunol. 122: 19–30. doi:10.1007/978-3-642-70740-7_3.
- ↑ Eicher, E. M.; Beamer, W. G.; Washburn, L. L.; Whitten, W. K. (1980). "A cytogenetic investigation of inherited true hermaphroditism in BALB/cWt mice". Cytogenet. Cell Genet. 28: 104–115. doi:10.1159/000131518.
- ↑ Mouse: Northwestern University Ecodome Information Page
- 1 2 3 4 "Guidelines for Selecting Route and Needle Size". Duke University and Medical Center - Animal Care & Use Program. Archived from the original on 17 Jan 2005. Retrieved April 2011. Check date values in:
|access-date=(help) - ↑ A Compendium of Drugs Used for Laboratory Animal Anesthesia, Analgesia, Tranquilization and Restraint at Drexel University College of Medicine. Retrieved April 2011
- 1 2 Guidelines for Systemic Anesthetics (Mouse) From Duke University and Medical Center - Animal Care & Use Program. Retrieved April 2011
- ↑ "Euthanasia". Basic Biomethodology for Laboratory Mice. Retrieved 2012-10-17.
- ↑ 2013 AVMA Guidelines for the Euthanasia of Animals
- ↑ Ng, TFF; Kondov, NO; Hayashimoto, N; Uchida, R; Cha, Y; et al. (2013). "Identification of an astrovirus commonly infecting laboratory mice in the US and Japan". PLoS ONE. 8 (6): e66937. doi:10.1371/journal.pone.0066937.
- ↑ Anon. "Animal Research". Policy issues. Society of Biology. Retrieved 18 October 2014.
- ↑ "Annual Statistics of Scientific Procedures on Living Animals: Great Britain 2012" (PDF). Home Office (UK). 2013. Retrieved July 30, 2013.
- ↑ Anon (2014). "Annual Statistics of Scientific Procedures on Living Animals Great Britain 2013". National statistics. Home Office. p. 26. Retrieved 18 October 2014.
- ↑ "Office of Laboratory Animal Welfare: PHS Policy on Humane Care and Use of Laboratory Animals". Grants.nih.gov. Retrieved 2010-07-29.
- 1 2 Kolata, Gina (11 February 2013). "Mice Fall Short as Test Subjects for Some of Humans' Deadly Ills". New York Times. Retrieved 6 August 2015.
- 1 2 3 Seok; et al. (7 January 2013). "Genomic responses in mouse models poorly mimic human inflammatory diseases". Proceedings of the National Academy of Sciences. Retrieved 6 August 2015.
- ↑ Bart van der Worp, H (30 March 2010). "Can Animal Models of Disease Reliably Inform Human Studies?". PLOS Medicine. 2: 1385. doi:10.1371/journal.pmed.1000245. PMC 1690299
 . PMID 1000245. Retrieved 6 August 2015.
. PMID 1000245. Retrieved 6 August 2015. - 1 2 3 Gawrylewski, Andrea (1 July 2007). "The Trouble With Animal Models". The Scientist. Retrieved 6 August 2015.
- ↑ Benatar, M (April 2007). "Lost in translation: Treatment trials in the SOD1 mouse and in human ALS". Neurobiology of Disease. 26 (1): 1–13. doi:10.1016/j.nbd.2006.12.015. Retrieved 6 August 2015.
- 1 2 Check Hayden, Erika (26 March 2014). "Misleading mouse studies waste medical resources". Nature. Retrieved 6 August 2015.
- 1 2 Perrin, Steve (26 March 2014). "Preclinical research: Make mouse studies work". Nature. Retrieved 6 August 2015.
- ↑ Cavanaugh, Sarah; Pippin, John; Bernard, Neal (10 April 2013). "Animal models of Alzheimer disease: historical pitfalls and a path forward1" (PDF). ALTEX. 31 (3): 279–302. doi:10.14573/altex.1310071. Retrieved 6 August 2015.
- ↑ Roep, Bart; Atkinson, Mark; von Herrath, Matthias (November 2004). "Satisfaction (not) guaranteed: re-evaluating the use of animal models in type 1 diabetes". Nature Immunology. 4: 989–997. doi:10.1038/nri1502. Retrieved 6 August 2015.
- ↑ Charukeshi Chandrasekera, P; Pippin, John (21 November 2013). "Of Rodents and Men: Species-Specific Glucose Regulation and Type 2 Diabetes Research" (PDF). ALTEX. 31: 157–176. doi:10.14573/altex.1309231. Retrieved 6 August 2015.
- ↑ Glenn Begley, C; Ellis, L (29 March 2012). "Drug development: Raise standards for preclinical cancer research". Nature. 483: 531–533. doi:10.1038/483531a. PMID 22460880. Retrieved 6 August 2015.
- ↑ Voskoglou-Nomikos, T; Pater, J; Seymour, L (15 September 2003). "Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models" (PDF). Clinical Cancer Research. 9: 4227– 4239. Retrieved 6 August 2015.
- ↑ Dennis, C (17 August 2006). "Cancer: off by a whisker". Nature. 442 (7104): 739–41. doi:10.1038/442739a. PMID 16915261.
- ↑ Garber, K (6 September 2006). "Debate Grows Over New Mouse Models of Cancer". Journal of the National Cancer Institute. 98 (17): 1176–8. doi:10.1093/jnci/djj381. PMID 16954466.
- ↑ Begley, Sharon (5 September 2008). "Rethinking the war on cancer". Newsweek. Retrieved 6 August 2015.
- 1 2 Bolker, Jessica (1 November 2012). "There's more to life than rats and flies". Nature. Retrieved 6 August 2015.
- ↑ Cressey, Daniel (2 March 2010). "Fat rats skew research results". Nature. 464 (19). doi:10.1038/464019a. Retrieved 6 August 2015.
- ↑ Balcomb, J; Barnard, N; Sandusky, C (November 2004). "Laboratory routines cause animal stress.". Contemporary Topics in Laboratory Animal Science. 43 (6): 42–51. PMID 15669134.
- ↑ Murgatroyd, C; et al. (8 November 2009). "Dynamic DNA methylation programs persistent adverse effects of early-life stress". Nature Neuroscience. 12: 1559–1566. doi:10.1038/nn.2436. PMID 19898468. Retrieved 6 August 2015.
Further reading
- Musser, G.G.; Carleton, M.D. (2005). "Superfamily Muroidea". In Wilson, D.E.; Reeder, D.M. Mammal Species of the World: a taxonomic and geographic reference (3rd ed.). Baltimore: Johns Hopkins University Press. pp. 894–1531. ISBN 978-0-8018-8221-0.
- Nyby J. (2001). "Ch. 1 Auditory communication in adults". In Willott, James F. Handbook of Mouse Auditory Research: From Behavior to Molecular Biology. Boca Raton: CRC Press. pp. 3–18.
External links
| Wikimedia Commons has media related to Mus musculus. |
| Wikispecies has information related to: Mus musculus |
Taxonomy
Genetics
- "Laboratory mouse". National Center for Biotechnology Information (NCBI).
- Ensembl Mus musculus genome browser, from the Ensembl Project
- Vega Mus musculus genome browser, includes NOD mouse sequence and annotation
Media
- Pictures, movies and applets showing the anatomy of Mus musculus, from www.digimorph.org
- Michael Purdy: "Researchers add mice to list of creatures that sing in the presence of mates"-Study of male mouse "song" with mouse song recording (MP3), by Washington University Medical School
- Arkive Photographs.Short text.
- High-Resolution Brain Maps and Brain Atlases of Mus musculus
Further reading
- Biology of the Mouse, from the Louisiana Veterinary Medical Association
- Nature Mouse Special 2002
- Biology of Laboratory Rodents by David G. Besselsen