LR-5182
 | |
| Identifiers | |
|---|---|
| |
| Synonyms | (cis)-LR-5182, (cis)-LR-5182 hydrochloride |
| CAS Number | HCl: 62373-97-1 |
| PubChem (CID) |
44024 HCl: CID 44023 |
| ChemSpider |
40076 HCl: 40075 |
| ChEMBL | CHEMBL322067 |
| Chemical and physical data | |
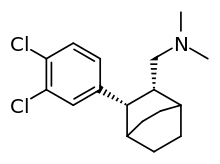
| Formula | C17H23Cl2N |
| Molar mass | 312.28 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
LR-5182 is a stimulant drug which acts as a norepinephrine–dopamine reuptake inhibitor, structurally related to the better known drug fencamfamine.[1][2][3] It was developed by the pharmaceutical company Eli Lilly in the 1970s, and researched for potential use as an antidepressant, although never marketed. LR-5182 has two stereoisomers, both of which are active, although one isomer blocks reuptake of only dopamine and noradrenaline, while the other blocks reuptake of serotonin as well.[4]
While LR-5182 itself never proceeded beyond initial animal studies, discovery of monoamine reuptake inhibition activity and stimulant effects in drugs of this type has subsequently led to the development of many other stimulant drugs of related chemical structure, primarily developed as potential antidepressants,[5] or as substitute drugs for the treatment of cocaine abuse.[6][7]
References
- ↑ David T. Wong; Frank P. Bymaster (September 1978). "An inhibitor of dopamine uptake, LR5182, CIS-3-(3,4-dichlorophenyl)-2-N, N-dimethylaminomethyl-bicyclo-[2,2,2]-octane, hydrochloride". Life Sciences. 23 (10): 1041–1047. doi:10.1016/0024-3205(78)90664-1. PMID 713683.
- ↑ R.W. Fuller; K.W. Perry; H.D. Snoddy (May 1979). "In vivo effects of LR5182, cis-3-(3,4-dichlorophenyl)-2-N,N-dimethylaminomethyl-bicyclo-[2,2,2]-octane hydrochloride, an inhibitor of uptake into dopamine and norepinephrine neurons". Neuropharmacology. 18 (5): 497–501. doi:10.1016/0028-3908(79)90076-5. PMID 460546.
- ↑ David T. Wong; Frank P. Bymaster; Leroy R. Reid (June 1980). "Competitive Inhibition of Catecholamine Uptake in Synaptosomes of Rat Brain by Rigid Bicyclo-Octanes". Journal of Neurochemistry. 34 (6): 1453–1458. doi:10.1111/j.1471-4159.1980.tb11225.x. PMID 7381469.
- ↑ Susan Wedley; Joy L. Howard; Bruce T. Large; Ian A. Pullar (1978). "The inhibition of monoamine uptake into rat brain synaptosomes by selected bicyclo-octanes and an analogous bicyclo-octene". Biochemical Pharmacology. 27 (24): 2907–2909. doi:10.1016/0006-2952(78)90207-1. PMID 736983.
- ↑ Lorraine Axford; John R. Boot; Terrence M. Hotten; Martine Keenan; Fionna M. Martin; Sandra Milutinovic; Nick A. Moore; Michael F. O'Neill; Ian A. Pullar; David E. Tupper; Kristel R. Van Belle; Vincent Vivien (October 2003). "Bicyclo[2.2.1]heptanes as novel triple re-uptake inhibitors for the treatment of depression". Bioorganic & Medicinal Chemistry Letters. 13 (19): 3277–3280. doi:10.1016/S0960-894X(03)00660-7. PMID 12951108.
- ↑ Howard M. Deutsch; David M. Collard; Liang Zhang; Kikue S. Burnham; Abhay K. Deshpande; Stephan G. Holtzman; Margaret M. Schweri (March 1999). "Synthesis and Pharmacology of Site-Specific Cocaine Abuse Treatment Agents: 2-(Aminomethyl)-3-phenylbicyclo[2.2.2]- and -[2.2.1]alkane Dopamine Uptake Inhibitors". Journal of Medicinal Chemistry. 42 (5): 882–895. doi:10.1021/jm980566m. PMID 10072685.
- ↑ Sahar Javanmard; Howard M. Deutsch; David M. Collard; Michael J. Kuhar; Margaret M. Schweri (November 1999). "Synthesis and Pharmacology of Site-Specific Cocaine Abuse Treatment Agents: 2-Substituted-6-amino-5-phenylbicyclo[2.2.2]octanes". Journal of Medicinal Chemistry. 42 (23): 4836–4843. doi:10.1021/jm990306k. PMID 10579846.