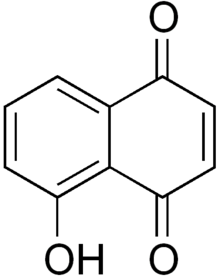
Juglone
 | |
 | |
| Names | |
|---|---|
| IUPAC name
5-Hydroxy-1,4-naphthalenedione | |
Other names
| |
| Identifiers | |
| 481-39-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 3674 |
| ECHA InfoCard | 100.006.880 |
| PubChem | 3806 |
| RTECS number | QJ5775000 |
| UNII | W6Q80SK9L6 |
| |
| |
| Properties | |
| C10H6O3 | |
| Molar mass | 174.16 g·mol−1 |
| Appearance | Yellow solid |
| Melting point | 162 to 163 °C (324 to 325 °F; 435 to 436 K) |
| Slightly sol. | |
| Hazards | |
| R-phrases | R25 |
| S-phrases | S28 S45 |
| Related compounds | |
| Related compounds |
quinone |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Juglone, also called 5-hydroxy-1,4-naphthalenedione (IUPAC) is an organic compound with the molecular formula C10H6O3. In the food industry, juglone is also known as C.I. Natural Brown 7 and C.I. 75500. It is insoluble in benzene but soluble in dioxane, from which it crystallizes as yellow needles. It is an isomer of lawsone, which is the staining compound in the henna leaf.
Juglone occurs naturally in the leaves, roots, husks, fruit (the epicarp), and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra), and is toxic or growth-stunting to many types of plants.[1] It is sometimes used as an herbicide, as a dye for cloth and inks, and as a coloring agent for foods and cosmetics.
History
The harmful effects of walnut trees on other plants have been observed for at least two millennia. The ancient civilizations of Greece and Rome used the walnut for its cytotoxic properties as did residents of the American South for easily gathering fish when they threw cut husks into the water with the fish.[2] However, juglone was not isolated until the 1850s. Two men, A. Vogel Jr. and C. Reischauer, were able to isolate the compound from the walnut tree in 1851. The compound was known as nucin at that time. Juglone was then synthesized and characterized for the first time in 1887 by A. Bernthsen and A. Semper. It was not until 1928 the compound was identified and confirmed to be toxic to other plants by E.F. Davis.
The use of walnut tree has historically been used within the field of traditional medicine. In America during the early 1900s, doctors prescribed juglone for the treatment of various skin diseases.[3]
Chemistry
Synthesis
Juglone is derived by oxidation of 1,5-dihydroxynaphthalene.[4] It can be also be obtained by oxidations of 5,8-dihydroxy-1-tetralone with silver oxide (Ag2O), manganese dioxide (MnO2), or 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ).[5]
Extraction
Juglone has been extracted from the husk of walnut fruit of which it contains 2-4% by fresh weight.[6][7]
Biological effects
Juglone is an example of allelopathic compound, a substance that is produced by a plant to stunt the growth of another. Landscapers have long known that gardening underneath or near black walnut trees can be difficult. Juglone exerts its effect by inhibiting certain enzymes needed for metabolic function. A number of plants and trees are resistant to juglone including some species of maple (Acer), birch (Betula), and beech (Fagus).
It is highly toxic to many insect herbivores. Some of them, however, are capable of detoxification of juglone (and related naphthoquinones) to non-toxic 1,4,5-trihydroxynaphthalene. It has also shown anthelmintic (expelling parasitic worms) activity on mature and immature Hymenolepis nana in mice.[8] Napthoquinonic compounds also exhibit antimicrobial activity.[9][10][11]
Uses
Juglone is occasionally used as a herbicide. Traditionally, juglone has been used as a natural dye for clothing and fabrics, particularly wool, and as ink. Because of its tendency to create dark orange-brown stains, juglone has also found use as a coloring agent for foods and cosmetics, such as hair dyes.
Juglone is currently being studied for its anticancer properties.[12] One of the potential pathways through which juglone achieves its anticancer properties is through the formation of the semiquinone radical; the semiquinone radical causes superoxide anion radicals to form which can lead to apoptosis when present in large concentrations.[13] This conversion from juglone to semiquinone radical that causes the superoxide anion radical to form takes place in the mitochondria as well as the cytosol.[14]
Spectral data
The spectral data for juglone confirms its bicyclic structure which contains a hydroxyl group as well as two carbonyl groups. The IR for juglone shows peaks at 3400 cm-1, 1662 cm-1, and 1641 cm-1 which are characteristic of the hydroxyl and carbonyl groups.[15] The 13C NMR shows 10 peaks indicating the correct number of unique carbon atoms in the molecule as well as peaks at 160.6 ppm, 183.2 ppm, and 189.3 ppm for the carbon attached to the hydroxyl group and the two carbons part of the two carbonyl groups.[15][5]
References
- ↑ Juglone toxicity
- ↑ Soderquist, Charles J. (1973). "Juglone and allelopathy". Journal of Chemical Education. 50 (11): 782–3. Bibcode:1973JChEd..50..782S. doi:10.1021/ed050p782. PMID 4747927.
- ↑ M. Strugstad (2012). "A Summary of Extraction, Synthesis, Properties, and Potential Uses of Juglone: A Literature Review". Journal of Ecosystems and Management. 13 (3): 72–82.
- ↑ Gerald Booth "Naphthalene Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_009.
- 1 2 J. Khalafy; J.M. Bruce (2002). "Oxidative dehydrogenation of 1-tetralones: Synthesis of juglone, naphthazarin, and α-hydroxyanthraquinones". Journal of Sciences, Islamic Republic of Iran. 13 (2): 131–139.
- ↑ Combes, M. R. (1907). "Bulletin de la Société chimique de France". Combes, Bull. Soc. Chim. (in French). 1 (4): 800–816. Retrieved 14 October 2016.
- ↑ Cosmulescu, Sina Niculina; Trandafir, Ion; Achim, Gheorghe; Botu, Mihai; Baciu, Adrian; Gruia, Marius (15 June 2010). "Phenolics of Green Husk in Mature Walnut Fruits". Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 38 (1): 53–56. doi:10.15835/nbha3814624 (inactive 2016-10-24). ISSN 1842-4309. Retrieved 11 October 2016.
- ↑ Dama L.B.; Jadhav B.V. (1997). "Anthelmintic effect of Juglone on mature and Immature Hymenolepis nana in mice". Riv. Di Parassitol. 2: 301–302.
- ↑ Dama L.B.; Poul B.N.; Jadhav B.V. (1998). "Antimicrobial activity of Napthoquinonic compounds". J. Ecotoxicol.Environ. Monit. 8: 213–215.
- ↑ Dama L.B.; Poul B.N.; Jadhav B.V; Hafeez MD. (1999). "Effect of "Juglone" on Development of the plant parasitic nematode (Meloidogyne Spp.) on Arachis hypogaea L.". J. Ecotoxicol. Environ. Monit. 9: 73–75.
- ↑ Dama L.B. (2002). "Effect of naturally occurring napthoquinones on root- knot nematode Meloidogyne spp.". Indian Phytopathology. 55 (1): 67–69.
- ↑ Chen, L; Na-Shun, B. Y.; Zhang, J; Yu, J; Gu, W. W. (Jun 2009). "Effect of juglone on the ultrastructure of human liver cancer BEL-7402 cells". Nan Fang Yi Ke Da Xue Xue Bao. 29 (6): 1208–11. PMID 19726363.
- ↑ Ji, Yu-Bin; Qu, Zhong-Yuan; Zou, Xiang (2011). "Juglone-induced apoptosis in human gastric cancer SGC-7901 cells via the mitochondrial pathway". Experimental and Toxicologic Pathology. 63 (1–2): 69–78. doi:10.1016/j.etp.2009.09.010. PMID 19815401.
- ↑ Inbaraj, J. Johnson; Chignell, Colin F. (2004). "Cytotoxic Action of Juglone and Plumbagin: A Mechanistic Study Using HaCaT Keratinocytes". Chemical Research in Toxicology. 17 (1): 55–62. doi:10.1021/tx034132s. PMID 14727919.
- 1 2 Suchard, Oliver; Kane, Ronan; Roe, Bernard J.; Zimmermann, Elmar; Jung, Christian; Waske, Prashant A.; Mattay, Jochen; Oelgemöller, Michael (2006). "Photooxygenations of 1-naphthols: An environmentally friendly access to 1,4-naphthoquinones". Tetrahedron. 62 (7): 1467. doi:10.1016/j.tet.2005.11.021.