Ixodes scapularis
| Ixodes scapularis | |
|---|---|
 | |
| Adult female deer tick | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Chelicerata |
| Class: | Arachnida |
| Subclass: | Acari |
| Superorder: | Parasitiformes |
| Order: | Ixodida |
| Family: | Ixodidae |
| Genus: | Ixodes |
| Species: | I. scapularis |
| Binomial name | |
| Ixodes scapularis Say, 1821 | |
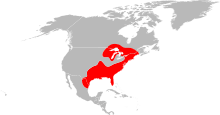 | |
Ixodes scapularis is commonly known as the deer tick or blacklegged tick (although some people reserve the latter term for Ixodes pacificus, which is found on the West Coast of the USA), and in some parts of the USA as the bear tick.[1] It is a hard-bodied tick (family Ixodidae) of the eastern and northern Midwestern United States. It is a vector for several diseases of animals, including humans (Lyme disease, babesiosis, anaplasmosis, Powassan virus disease, etc.) and is known as the deer tick owing to its habit of parasitizing the white-tailed deer. It is also known to parasitize mice,[2] lizards,[3] migratory birds,[4] etc. especially while the tick is in the larval or nymphal stage.

Description
The image shown at the upper right—and in fact, most images of Ixodes scapularis that are commonly available—show an adult that is unengorged, that is, an adult that has not had a blood meal. This is natural, since ticks are generally removed immediately upon discovery to minimize the chance of disease. However, the abdomen that holds blood is so much larger when engorged and looks so different from the rest of the tick that it would be easy to assume that an engorged specimen of Ixodes scapularis is an entirely different tick (see photo below).
When the deer tick has consumed a blood meal, its abdomen will be a light grayish-blue color, whereas the tick itself is chiefly black. In identifying an engorged tick, it is helpful to concentrate on the legs and upper part of the body.
Behavior
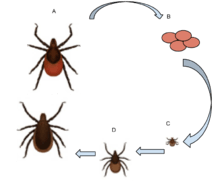
I. scapularis has a two-year life cycle, during which time it passes through three stages: larva, nymph, and adult. The tick must take a blood meal at each stage before maturing to the next. Deer tick females latch onto a host and drink its blood for four to five days. Deer are the preferred host of the deer tick, but it is also known to feed on small rodents.[5] After she is engorged, the tick drops off and overwinters in the leaf litter of the forest floor. The following spring, the female lays several hundred to a few thousand eggs in clusters.[6] Transtadial (between tick stages) passage of Borrelia burgdorferi is common. Vertical passage (from mother to egg) of Borrelia is uncommon.
Ticks are very hardy creatures and I. scapularis is no exception. They will be active even after a moderate to severe frost, as daytime temperatures can warm them enough to keep them actively searching for a host. In the spring, they can be one of the first invertebrates to become active. Deer ticks can be quite numerous and seemingly gregarious in areas where they are found.
As disease vector


Ixodes scapularis is the main vector of Lyme disease in North America.[7] It can also transmit other Borrelia species, including Borrelia miyamotoi.[8] Ticks that transmit B. burgdorferi to humans can also carry and transmit several other parasites, such as Theileria microti and Anaplasma phagocytophilum, which cause the diseases babesiosis and human granulocytic anaplasmosis (HGA), respectively.[9] Among early Lyme disease patients, depending on their location, 2%–12% will also have HGA and 2%–40% will have babesiosis.[10]

Co-infections complicate Lyme symptoms, especially diagnosis and treatment. It is possible for a tick to carry and transmit one of the co-infections and not Borrelia, making diagnosis difficult and often elusive. The Centers for Disease Control's emerging infectious diseases department did a study in rural New Jersey of 100 ticks, and found 55% of the ticks were infected with at least one of the pathogens.[11]
Although they are the preferred mammalian hosts, deer cannot transmit Borrelia spirochetes to ticks. Ticks acquire Lyme disease microbes by feeding on infected mice and other small rodents.[5]
One of the keys of the success of I. scapularis as a Borrelia vector relies on its ability to limit the proliferation of the spirochaete. This is due to the activity of domesticated amidase effector (dae) genes. Dae genes are a family of horizontally acquired genes related to type VI secretion amidase effector (tae) genes in certain bacteria which encode toxins honed to mediate interbacterial antagonism. Once transferred to eukaryotes tae genes confer novel antibacterial capabilities;[12] this provides a selective advantage to the tick and to other eukaryotes also: tae genes have been transferred from bacteria to eukaryotes at least in six independent events. In particular, I. scapularis have inherited the dae 2 family from a common ancestor between ticks and mites.[12] The product of dae2 expression has been shown to degrade bacterial peptidoglycan of different species and particularly from B. burgdorferi, but does not limit initial acquisition of the bacterium by the tick. Dae2 contributes to the innate ability of I. scapularis to control B. burgdorferi levels after its acquisition. This has potential ramifications for Lyme disease transmission, as spirochaete load in the tick can influence transmission efficiency.[12]
Predators
Guineafowl, chickens, and fire ants are known predators of ticks. None has been proven to be effective in populations on a large scale, but anecdotal evidence supports localized control of tick populations.
Genome sequencing
| NCBI genome ID | 523 |
|---|---|
| Ploidy | diploid |
| Genome size | 1,765.38 Mb |
| Number of chromosomes | 15 pairs |
| Year of completion | 2008 |
The genome of I. scapularis has been sequenced.[13]
See also
References
- ↑ Drummond, Roger (2004). Ticks and What You Can Do about Them (3rd ed.). Berkeley, California: Wilderness Press. p. 23. ISBN 0-89997-353-1.
- ↑ Mannelli, A; Kitron, U; Jones, C. J.; Slajchert, T. L. (1994). "Influence of season and habitat on Ixodes scapularis infestation on white-footed mice in northwestern Illinois". The Journal of Parasitology. 80 (6): 1038–42. doi:10.2307/3283457. PMID 7799148.
- ↑ Levine, J. F.; Apperson, C. S.; Howard, P; Washburn, M; Braswell, A. L. (1997). "Lizards as hosts for immature Ixodes scapularis (Acari: Ixodidae) in North Carolina". Journal of medical entomology. 34 (6): 594–8. doi:10.1093/jmedent/34.6.594. PMID 9439111.
- ↑ Ogden NH, Lindsay LR, Hanincová K, Barker IK, Bigras-Poulin M, Charron DF, Heagy A, Francis CM, O'Callaghan CJ, Schwartz I, Thompson RA (2008). "Role of migratory birds in introduction and range expansion of Ixodes scapularis ticks and of Borrelia burgdorferi and Anaplasma phagocytophilum in Canada". Appl. Environ. Microbiol. 74: 1780–90. doi:10.1128/AEM.01982-07. PMC 2268299
 . PMID 18245258.
. PMID 18245258. - 1 2 "Westport Weston Health District". 2004. Retrieved 2013-09-26.
- ↑ Suzuki, David; Grady, Wayne (2004). Tree: A Life Story. Vancouver: Greystone Books. p. 110. ISBN 1-55365-126-X.
- ↑ Brownstein, John S.; Holford, Theodore R.; Fish, Durland (2005). "Effect of Climate Change on Lyme Disease Risk in North America". EcoHealth. 2 (1): 38–46. doi:10.1007/s10393-004-0139-x. PMC 2582486
 . PMID 19008966.
. PMID 19008966. - ↑ McNeil, Donald (19 September 2011). "New Tick-Borne Disease Is Discovered". The New York Times. pp. D6. Retrieved 20 September 2011.
- ↑ Steere AC (July 2001). "Lyme disease". New England Journal of Medicine. 345 (2): 115–25. doi:10.1056/NEJM200107123450207. PMID 11450660.
- ↑ G. P. Wormser (June 2006). "Clinical practice. Early Lyme disease". New England Journal of Medicine. 354 (26): 2794–801. doi:10.1056/NEJMcp061181. PMID 16807416.
- ↑ Varde S, Beckley J, Schwartz I (1998). "Prevalence of tick-borne pathogens in Ixodes scapularis in a rural New Jersey County". Emerging Infectious Diseases. 4 (1): 97–99. doi:10.3201/eid0401.980113. PMC 2627663
 . PMID 9452402.
. PMID 9452402. - 1 2 3 Seemay Chou; Matthew D. Daugherty; S. Brook Peterson; Jacob Biboy; Youyun Yang; Brandon L. Jutras; Lillian K. Fritz-Laylin; Michael A. Ferrin; Brittany N. Harding; Christine Jacobs-Wagner; X. Frank Yang; Waldemar Vollmer; Harmit S. Malik; Joseph D. Mougous (2014). "Transferred interbacterial antagonism genes augment eukaryotic innate immune function". Nature. 518: 98–101. doi:10.1038/nature13965.
- ↑ Ixodes scapularis genome sequence at VectorBase
See also
External links
- Information on Tick-Related Health Threats and Deer Tick Fact Sheet from the National Pest Management Association
- blacklegged tick, Ixodes scapularis on the UF / IFAS Featured Creatures Web site
- Ixodes scapularis, black-legged tick, deer tick overview as a vector for Lyme disease, developmental stages at MetaPathogen
- Ixodes scapularis genome sequence at VectorBase
- Powassan Virus: Transmission on the Centers for Disease Control and Prevention website.