Isopentenyl-diphosphate delta isomerase
| Isopentenyl-pyrophosphate delta isomerase 1 | |
|---|---|
| Identifiers | |
| Symbol | IDI1 |
| Entrez | 3422 |
| HUGO | 5387 |
| OMIM | 604055 |
| RefSeq | NM_004508 |
| UniProt | Q13907 |
| Other data | |
| EC number | 5.3.3.2 |
| Locus | Chr. 10 p15.3 |
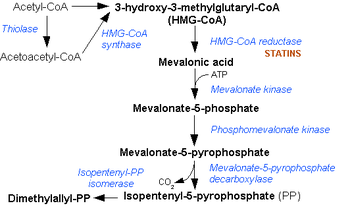
Isopentenyl pyrophosphate isomerase (IPP isomerase), also known as Isopentenyl-diphosphate delta isomerase,[1] is an isomerase that catalyzes the conversion of the relatively un-reactive isopentenyl pyrophosphate (IPP) to the more-reactive electrophile dimethylallyl pyrophosphate (DMAPP). This isomerization is a key step in the biosynthesis of isoprenoids through the mevalonate pathway.
Enzyme mechanism
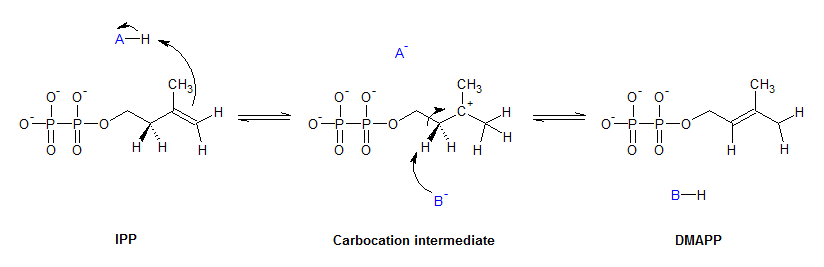
IPP isomerase catalyzes the isomerization of IPP to DMAPP by an antarafacial transposition of hydrogen.[2][3] The empirical evidence suggests that this reaction proceeds by a protonation/deprotonation mechanism, with the addition of a proton to the re-face of the inactivated C3-C4 double bond resulting in a transient carbocation intermediate.[4][5] The removal of the pro-R proton from C2 forms the C2-C3 double bond of DMAPP.
Enzyme structure

Crystallographic studies have observed that the active form of IPP isomerase is a monomer with alternating α-helices and β-sheets.[6][7] The active site of IPP isomerase is deeply buried within the enzyme and consists of a glutamic acid residue and a cysteine residue that interact with opposite sides of the IPP substrate, consistent with the antarafacial stereochemistry of isomerization.[6][8] The origin of the initial protonation step has not been conclusively established. Recent evidence suggests that the glutamic acid residue is involved in the protonating step despite the observation that its carboxylic acid side-chain is stabilized in its carboxylate form.[9] This discrepancy has been addressed by the discovery of a water molecule in the active site of human IPP isomerase, suggesting a mechanism where the glutamine residue polarizes the double bond of IPP and makes it more susceptible to protonation by water.[10]
IPP isomerase also requires a divalent cation to fold into its active conformation. The enzyme contains several amino acids, including the catalytic glutamate, that are involved in coordinating with Mg2+ or Mn2+.[6][11] The coordination of the metal cation to the glutamate residue stabilizes the carbiocation intermediate after protonation.
Biological function
The protonation of an inactivated double bond is rarely seen in nature, highlighting the unique catalytic mechanism of IPP isomerase. The isomerization of IPP to DMAPP is a crucial step in the synthesis of isoprenoids and isoprenoid-derivatives, compounds that play vital roles in the biosynthetic pathways of all living organisms.[12] Because of the importance of the melavonate pathway in isoprenoid biosynthesis, IPP isomerase is found in a variety of different cellular compartments, including plastids and mammalian mitochondria.[13]
Disease relevance
Mutations in IDI1, the gene that codes for IPP isomerase 1, have been implicated in decreased viability in a number of organisms, including the yeast Saccharomyces cerevisiae, the nematode Caenorhabditis elegans and the plant Arabidopsis thaliana.[14][15][16] While there have been no evidence directly implicating IDI1 mutations in human disease, genomic analysis has identified a copy-number gain near two IPP isomerase genes in a substantial proportion of patients with sporadic amyotrophic lateral sclerosis, suggesting that the isomerase may play a role in this disease.[17]
References
- ↑ "IDI1 - Isopentenyl-diphosphate Delta-isomerase - Saccharomyces cerevisiae (strain ATCC 204508 / S288c) (Baker's yeast) - IDI1 gene & protein". UniProt. Retrieved 6 June 2016.
- ↑ Cornforth JW, Cornforth RH, Popják G, Yengoyan L (Sep 1966). "Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate". The Journal of Biological Chemistry. 241 (17): 3970–3987. PMID 4288360.
- ↑ Cornforth RH, Popják G (1969). "Chemical syntheses of substrates of sterol biosynthesis". In Raymond BC. Methods in Enzymology. 15. Academic Press. pp. 359–390.
- ↑ Reardon JE, Abeles RH (Sep 1986). "Mechanism of action of isopentenyl pyrophosphate isomerase: evidence for a carbonium ion intermediate". Biochemistry. 25 (19): 5609–5616. doi:10.1021/bi00367a040. PMID 3022798.
- ↑ Street IP, Christensen DJ, Poulter CD (1990). "Hydrogen exchange during the enzyme-catalyzed isomerization of isopentenyl diphosphate and dimethylallyl diphosphate". Journal of the American Chemical Society. 112 (23): 8577–8578. doi:10.1021/ja00179a049.
- 1 2 3 Hall NR, Fish DE, Hunt N, Goldin RD, Guillou PJ, Monson JR (Jun 1992). "Is the relationship between angiogenesis and metastasis in breast cancer real?". Surgical Oncology. 1 (3): 223–229. doi:10.1016/0960-7404(92)90068-v. PMID 1285217.
- ↑ Zheng W, Sun F, Bartlam M, Li X, Li R, Rao Z (Mar 2007). "The crystal structure of human isopentenyl diphosphate isomerase at 1.7 A resolution reveals its catalytic mechanism in isoprenoid biosynthesis". Journal of Molecular Biology. 366 (5): 1447–1458. doi:10.1016/j.jmb.2006.12.055. PMID 17250851.
- ↑ Street IP, Coffman HR, Baker JA, Poulter CD (Apr 1994). "Identification of Cys139 and Glu207 as catalytically important groups in the active site of isopentenyl diphosphate:dimethylallyl diphosphate isomerase". Biochemistry. 33 (14): 4212–4217. doi:10.1021/bi00180a014. PMID 7908830.
- ↑ Wouters J, Oudjama Y, Barkley SJ, Tricot C, Stalon V, Droogmans L, Poulter CD (Apr 2003). "Catalytic mechanism of Escherichia coli isopentenyl diphosphate isomerase involves Cys-67, Glu-116, and Tyr-104 as suggested by crystal structures of complexes with transition state analogues and irreversible inhibitors". The Journal of Biological Chemistry. 278 (14): 11903–11908. doi:10.1074/jbc.M212823200. PMID 12540835.
- ↑ Zhang C, Liu L, Xu H, Wei Z, Wang Y, Lin Y, Gong W (Mar 2007). "Crystal structures of human IPP isomerase: new insights into the catalytic mechanism". Journal of Molecular Biology. 366 (5): 1437–1446. doi:10.1016/j.jmb.2006.10.092. PMID 17137593.
- ↑ Bonanno JB, Edo C, Eswar N, Pieper U, Romanowski MJ, Ilyin V, Gerchman SE, Kycia H, Studier FW, Sali A, Burley SK (Nov 2001). "Structural genomics of enzymes involved in sterol/isoprenoid biosynthesis". Proceedings of the National Academy of Sciences of the United States of America. 98 (23): 12896–12901. doi:10.1073/pnas.181466998. PMC 60796
 . PMID 11698677.
. PMID 11698677. - ↑ Bach TJ (Mar 1995). "Some new aspects of isoprenoid biosynthesis in plants--a review". Lipids. 30 (3): 191–202. doi:10.1007/BF02537822. PMID 7791527.
- ↑ Ramos-Valdivia AC, van der Heijden R, Verpoorte R (Dec 1997). "Isopentenyl diphosphate isomerase: a core enzyme in isoprenoid biosynthesis. A review of its biochemistry and function". Natural Product Reports. 14 (6): 591–603. doi:10.1039/np9971400591. PMID 9418296.
- ↑ Mayer MP, Hahn FM, Stillman DJ, Poulter CD (Sep 1992). "Disruption and mapping of IDI1, the gene for isopentenyl diphosphate isomerase in Saccharomyces cerevisiae". Yeast. 8 (9): 743–748. doi:10.1002/yea.320080907. PMID 1441751.
- ↑ Yochem J, Hall DH, Bell LR, Hedgecock EM, Herman RK (Apr 2005). "Isopentenyl-diphosphate isomerase is essential for viability of Caenorhabditis elegans". Molecular Genetics and Genomics. 273 (2): 158–166. doi:10.1007/s00438-004-1101-x. PMID 15765206.
- ↑ Okada K, Kasahara H, Yamaguchi S, Kawaide H, Kamiya Y, Nojiri H, Yamane H (Apr 2008). "Genetic evidence for the role of isopentenyl diphosphate isomerases in the mevalonate pathway and plant development in Arabidopsis". Plant & Cell Physiology. 49 (4): 604–616. doi:10.1093/pcp/pcn032. PMID 18303110.
- ↑ Kato T, Emi M, Sato H, Arawaka S, Wada M, Kawanami T, Katagiri T, Tsuburaya K, Toyoshima I, Tanaka F, Sobue G, Matsubara K (Nov 2010). "Segmental copy-number gain within the region of isopentenyl diphosphate isomerase genes in sporadic amyotrophic lateral sclerosis". Biochemical and Biophysical Research Communications. 402 (2): 438–442. doi:10.1016/j.bbrc.2010.10.056. PMID 20955688.
External links
- isopentenyldiphosphate delta-isomerase at the US National Library of Medicine Medical Subject Headings (MeSH)