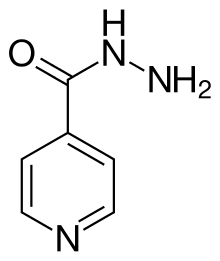
Isoniazid
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Hydra, Hyzyd, Isovit, other |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682401 |
| Pregnancy category |
|
| Routes of administration | by mouth, intramuscular, intravenous |
| ATC code | J04AC01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Very low (0-10%) |
| Metabolism | liver; CYP450: 2C19, 3A4 inhibitor |
| Biological half-life | 0.5-1.6h (fast acetylators), 2-5h (slow acetylators) |
| Excretion | urine (primarily), feces |
| Identifiers | |
| |
| Synonyms | Isonicotinic Acid Hydrazide, Isonicotinyl hydrazine, INHA, INH |
| CAS Number |
54-85-3 |
| PubChem (CID) | 3767 |
| DrugBank |
DB00951 |
| ChemSpider |
3635 |
| UNII |
V83O1VOZ8L |
| KEGG |
D00346 |
| ChEBI |
CHEBI:6030 |
| ChEMBL |
CHEMBL64 |
| NIAID ChemDB | 007657 |
| ECHA InfoCard | 100.000.195 |
| Chemical and physical data | |
| Formula | C6H7N3O |
| Molar mass | 137.139 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Isoniazid, also known as isonicotinylhydrazide (INH), is an antibiotic used as a first-line agent for the prevention and treatment of both latent and active tuberculosis.[1] It is effective against mycobacteria, particularly Mycobacterium tuberculosis. It is also active against some atypical types of mycobacteria, such as M. kansasii and M. xenopi.[2] Isoniazid is an organic compound that is available in tablet, syrup, and injectable forms.[3][4][5]
The most common side effect of isoniazid is an increase in blood levels of liver enzymes; however, it is usually harmless. Uncommon but more serious side effects include inflammation of nerves, which causes numbness in the arms or legs, and liver inflammation. Isoniazid blocks the formation of mycolic acids, which are essential parts of mycobacterial cell walls. Disruption of the mycobacterial cell wall results in cell death. Isoniazid acts on both intracellular and extracellular mycobacteria.[6]
Isoniazid was first made in 1952.[7] Three pharmaceutical companies unsuccessfully attempted to patent the drug at the same time,[8] the most prominent one being Roche, which launched its version, Rimifon, in 1952.[9] With the introduction of isoniazid, a cure for tuberculosis was first considered possible. It is available worldwide, is inexpensive, and is generally well tolerated. It is on the World Health Organization's List of Essential Medicines, a list of medicines that constitute the bare minimum for a basic health system.[10]
Medical uses
Isoniazid is approved for latent and active tuberculous infections. For the latter, it must be used in combination with other tuberculosis medications to prevent the development of drug resistance.
Isoniazid has been approved as prophylactic therapy for the following populations:
- People with HIV infection and a PPD reaction of at least 5 mm induration
- Contacts of people with tuberculosis and who have a PPD reaction at least 5 mm induration
- People whose PPD reactions convert from negative to positive in a two-year period – at least 10 mm induration for those up to 35 years of age, and at least 15 mm induration for those at least 35 years old
- People with pulmonary damage on their chest X-ray that is likely to be due to healed tuberculosis and also have a PPD reaction at least 5 mm induration
- Injection drug users whose HIV status is negative who have a PPD reaction at least 10 mm induration
- People with a PPD of greater than or equal to 10 mm induration who are foreign-born from high prevalence geographical regions, low-income populations, and patients residing in long-term facilities[11][12]
Special populations
It is recommended that women with active tuberculosis who are pregnant or breastfeeding take isoniazid. Preventive therapy should be delayed until after giving birth.[13] Nursing mothers excrete a relatively low and non-toxic concentration of INH in breast milk, and their babies are at low risk for side effects. Both pregnant women and infants being breastfed by mothers taking INH should take vitamin B6 in its pyridoxine form to minimize the risk of peripheral nerve damage.[14] Vitamin B6 is used to prevent isoniazid-induced B6 deficiency and neuropathy in people with a risk factor, such as pregnancy, lactation, HIV infection, alcoholism, diabetes, kidney failure, or malnutrition.[15]
People with liver dysfunction are at a higher risk for hepatitis caused by INH, and may need a lower dose.[13]
Levels of liver enzymes in the bloodstream should be frequently checked in daily alcohol drinkers, pregnant women, IV drug users, people over 35, and those who have chronic liver disease, severe kidney dysfunction, peripheral neuropathy, or HIV infection since they are more likely to develop hepatitis from INH.[13][16]
Side effects
Up to 20% of people taking isoniazid experience peripheral neuropathy when taking doses of 6 mg/kg of weight daily or higher.[17] Gastrointestinal reactions include nausea and vomiting.[11] Aplastic anemia, thrombocytopenia, and agranulocytosis due to lack of production of red blood cells, platelets, and white blood cells by the bone marrow, respectively can also occur.[11] Hypersensitivity reactions are also common and can present with a maculopapular rash and fever.[11]
Asymptomatic elevation of serum liver enzyme concentrations occurs in 10% to 20% of people taking INH, and liver enzyme concentrations usually return to normal even when treatment is continued.[18] Isoniazid has a boxed warning for severe and sometimes fatal hepatitis, which is age-dependent at a rate of 0.3% in people 21 to 35 years old and over 2% in those over age 50.[11][19] Symptoms suggestive of liver toxicity include nausea, vomiting, abdominal pain, dark urine, right upper quadrant pain, and loss of appetite.[11] Black and Hispanic women are at higher risk for isoniazid-induced hepatotoxicity.[11] When it happens, isoniazid-induced liver toxicity has been shown to occur in 50% of patients within the first 2 months of therapy.[20]
Some recommend that liver function should be monitored carefully in all people receiving it,[21] but others recommend monitoring only in certain populations.[18][22][23]
Headache, poor concentration, weight gain, poor memory, insomnia, and depression have all been associated with isoniazid use.[24] All patients and healthcare workers should be aware of these serious side effects, especially if suicidal ideation or behavior are suspected.[24][25][26]
Isoniazid is associated with pyridoxine deficiency due to the increased excretion of pyridoxine. Pyridoxal phosphate (a derivative of pyridoxine) is required for d-aminolevulinic acid synthase, the enzyme responsible for the rate-limiting step in heme synthesis. Therefore, isoniazid-induced pyridoxine deficiency causes insufficient heme formation in early red blood cells, leading to sideroblastic anemia.[15]
Drug interactions
People taking isoniazid and acetaminophen are at risk of acetaminophen toxicity. Isoniazid is thought to induce a liver enzyme which causes a larger amount of acetaminophen to be metabolized to a toxic form.[27][28]
Isoniazid decreases the metabolism of carbamazepine, thus slowing down its clearance from the body. People taking carbamazepine should have their carbamazepine levels monitored and, if necessary, have their dose adjusted accordingly.[29]
It is possible that isoniazid may decrease the serum levels of ketoconazole after long term treatment. This is seen with the simultaneous use of rifampin, isoniazid, and ketoconazole.<[30]
Isoniazid may increase the amount of phenytoin in the body. The doses of phenytoin may need to be adjusted when given with isoniazid.[31][32]
Isoniazid may increase the plasma levels of theophylline. There are some cases of theophylline slowing down isoniazid elimination. Both theophylline and isoniazid levels should be monitored.[33]
Valproate levels may increase when taken with isoniazid. Valproate levels should be monitored and its dose adjusted if necessary.[31]
Mechanism of action
Isoniazid is a prodrug and must be activated by a bacterial catalase-peroxidase enzyme in Mycobacterium tuberculosis called KatG.[34] KatG couples the isonicotinic acyl with NADH to form isonicotinic acyl-NADH complex. This complex binds tightly to the enoyl-acyl carrier protein reductase known as InhA, thereby blocking the natural enoyl-AcpM substrate and the action of fatty acid synthase. This process inhibits the synthesis of mycolic acids, which are required components of the mycobacterial cell wall. A range of radicals are produced by KatG activation of isoniazid, including nitric oxide,[35] which has also been shown to be important in the action of another antimycobacterial prodrug pretomanid.[36]
Isoniazid is bactericidal to rapidly dividing mycobacteria, but is bacteriostatic if the mycobacteria are slow-growing.[37] It inhibits the cytochrome P450 system and hence acts as a source of free radicals.[38]
Metabolism
Isoniazid reaches therapeutic concentrations in serum, cerebrospinal fluid, and within caseous granulomas. It is metabolized in the liver via acetylation into acetylhydrazine. Two forms of the enzyme are responsible for acetylation, so some patients metabolize the drug more quickly than others. Hence, the half-life is bimodal, with "slow acetylators" and "fast acetylators". A graph of number of people versus time shows peaks at one and three hours. The height of the peaks depends on the ethnicities of the people being tested. The metabolites are excreted in the urine. Doses do not usually have to be adjusted in case of renal failure.
History
The drug was first tested at Many Farms, a Navajo community, due to the Navajo reservation's tuberculosis problem and because the population had not previously been treated with streptomycin, the main tuberculosis treatment at the time.[39]
Preparation
Isoniazid is manufactured using 4-cyanopyridine and hydrazine hydrate.[40] The former reagent is produced via the ammoxidation of 4-methylpyridine.[41]
In another method, isoniazid was claimed to have been made from citric acid starting material.[42]
Brand names
Hydra, Hyzyd, Isovit, Laniazid, Nydrazid, Rimifon, and Stanozide.[43]
Other uses
Chromatography
Isonicotinic acid hydrazide is also used in chromatography to differentiate between various degrees of conjugation in organic compounds barring the ketone functional group.[44] The test works by forming a hydrazone which can be detected by its bathochromic shift.
References
- ↑ "Tuberculosis - TB Guidelines - Treatment". cdc.gov. Centers for Disease Control. Retrieved 22 February 2016.
- ↑ Berning S.E., Peloquin C.A. Antimycobacterial agents: Isoniazid. In: Antimicrobial Therapy and Vaccines, Yu V., Merigan T., Barriere S. (eds.) Williams and Wilkins, Baltimore 1998.
- ↑ Isoniazid [package insert]. Eatontown, NJ: West-Ward Pharmaceutical Corp; 2014.
- ↑ Isoniazid [package insert]. Atlanta, GA: Mikart, Inc; 2015
- ↑ Isoniazid [package insert]. Princeton, NJ: Sandoz, Inc; 2009.
- ↑ Lei, Benfang; Wei, Chih-Jen; Tu, Shiao-Chun (2000-01-28). "Action Mechanism of Antitubercular Isoniazid: Activation by Mycobacterium tuberculosis KatG, Isolation, and Characterization of InhA Inhibitro". Journal of Biological Chemistry. 275 (4): 2520–2526. doi:10.1074/jbc.275.4.2520. ISSN 0021-9258.
- ↑ Walker, S. R. (2012). Trends and Changes in Drug Research and Development. Springer Science & Business Media. p. 109. ISBN 9789400926592.
- ↑ Hans L. Riede (2009). "Fourth-generation fluoroquinolones in tuberculosis". Lancet. 373 (9670): 1148–1149. doi:10.1016/S0140-6736(09)60559-6. PMID 19345815.
- ↑ Roche USA
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 2 February 2015.
- 1 2 3 4 5 6 7 "Isoniazid (package insert)".
- ↑ "The Use of Preventive Therapy for Tuberculosis Infection in the United States - Recommendations of the Advisory Committee for Elimination of Tuberculosis". Morbidity and Mortality Weekly Report. 39 (RR-8): 9–12. May 18, 1990. Retrieved 22 February 2016.
- 1 2 3 https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=5ab01773-d1e4-4b87-884a-9e5b3f13bdcb
- ↑ Bothamley, G. (2001). "Drug treatment for tuberculosis during pregnancy: safety considerations". Drug safety. 24 (7): 553–565. doi:10.2165/00002018-200124070-00006. PMID 11444726.
- 1 2 Steichen, O.; Martinez-Almoyna, L.; De Broucker, T. (April 2006). "Isoniazid induced neuropathy: consider prevention". Revue des maladies respiratoires. 23 (2 Pt 1): 157–160. PMID 16788441.
- ↑ Saukkonen, J.J.; Cohn, D.L.; Jasmer, R.M. (October 15, 2006). "An official ATS statement: hepatotoxicity of antituberculosis therapy". American Journal of Respiratory and Critical Care Medicine. 174 (8): 935–952. doi:10.1164/rccm.200510-1666ST. PMID 17021358.
- ↑ Alldredge, Brian (February 12, 2013). Applied Therapeutics. ISBN 9781609137137.
- 1 2 "Latent Tuberculosis Infection: A Guide for Primary Health Care Providers". cdc.gov. Center for Disease Control. Retrieved 25 March 2016.
- ↑ Trevor, A. & Katzung, B. (2013). Katzung & Trevor's Pharmacology: examination & board review (10th ed., p. 417). New York. McGraw-Hill Medical, Lange.
- ↑ "Isoniazid UpToDate".
- ↑ "DailyMed - Isoniazid - isoniazid tablet". dailymed.nlm.nih.gov. Retrieved 2015-11-05.
- ↑ "Treatment of Tuberculosis - Guidelines (4th ed.)" (PDF). who.int. World Health Organization. Retrieved 25 March 2016.
- ↑ "Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Joint Tuberculosis Committee of the British Thoracic Society". Thorax. 53 (7): 536–548. July 1998. doi:10.1136/thx.53.7.536. PMC 1745276
 . PMID 9797751.
. PMID 9797751. - 1 2 Alao A.O.; Yolles J.C. (September 1998). "Isoniazid-induced psychosis". Annals of Pharmacotherapy. 32 (9): 889–891. doi:10.1345/aph.17377. PMID 9762376.
- ↑ Iannaccone, R.; Sue, Y.J.; Avner, J.R. (2002). "Suicidal psychosis secondary to isoniazid". Pediatric Emergency Care. 18 (1): 25–27. doi:10.1097/00006565-200202000-00008. PMID 11862134.
- ↑ Pallone K.A.; Goldman M.P.; Fuller M.A. (February 1993). "Isoniazid-associated psychosis: case report and review of the literature". Annals of Pharmacotherapy. 27 (2): 167–170. PMID 8439690.
- ↑ Murphy, R.; Swartz, R.; Watkins, P.B. (November 15, 1990). "Severe acetaminophen toxicity in a patient receiving isoniazid". Annals of Internal Medicine. 113 (10): 799–800. doi:10.7326/0003-4819-113-10-799. PMID 2240884.
- ↑ Burk, R.F.; Hill, K.E.; Hunt Jr., R.W.; Martin, A.E. (July 1990). "Isoniazid potentiation of acetaminophen hepatotoxicity in the rat and 4-methylpyrazole inhibition of it". Research Communications in Chemical Pathology and Pharmacolog. 69 (1): 115–118. PMID 2218067.
- ↑ Fleenor, M.E.; Harden, J.W.; Curtis, G. (June 1991). "Interaction between carbamazepine and antituberculosis agents". Chest. 99 (6): 1554. doi:10.1378/chest.99.6.1554a. PMID 2036861.
- ↑ Baciewicz, A.M.; Baciewicz Jr., F.A. (September 13, 1993). "Ketoconazole and fluconazole drug interactions". Archives of Internal Medicine. 153 (17): 1970–1976. doi:10.1001/archinte.153.17.1970. PMID 8357281.
- 1 2 Jonville, A.P.; Gauchez, A.S.; Autret, E.; Billard, C.; Barbier, P.; Nsabiyumva, F.; Breteau, M. (1991). "Interaction between isoniazid and valproate: a case of valproate overdosage". European Journal of Clinical Pharmacology. 40 (2): 197–198. PMID 2065702.
- ↑ Bass, Jr., J.B.; Farer, L.S.; Hopewell, P.C.; O'Brien, R.; Jacobs, R.F.; Ruben, F.; Snider, Jr., D.E.; Thornton, G. (May 1994). "Treatment of tuberculosis and tuberculosis infection in adults and children. American Thoracic Society and The Centers for Disease Control and Prevention". Am J Respir Crit Care Med. 149 (5): 1359–1374. doi:10.1164/ajrccm.149.5.8173779. PMID 8173779.
- ↑ Höglund, P.; Nilsson, L.G.; Paulsen, O. (February 1987). "Interaction between isoniazid and theophylline". European Journal of Respiratory Diseases. 70 (2): 110–116. PMID 3817069.
- ↑ Suarez, J.; Ranguelova, K.; Jarzecki, A.A.; et al. (March 2009). "An oxyferrous heme/protein-based radical intermediate is catalytically competent in the catalase reaction of Mycobacterium tuberculosis catalase-peroxidase (KatG)". The Journal of Biological Chemistry. 284 (11): 7017–7029. doi:10.1074/jbc.M808106200. PMC 2652337
 . PMID 19139099.
. PMID 19139099. - ↑ Timmins, G.S.; Master, S; Rusnak, F.; Deretic, V. (August 2004). "Nitric oxide generated from isoniazid activation by KatG: source of nitric oxide and activity against Mycobacterium tuberculosis". Antimicrobial Agents and Chemotherapy. 48 (8): 3006–3009. doi:10.1128/AAC.48.8.3006-3009.2004. PMC 478481
 . PMID 15273113.
. PMID 15273113. - ↑ Singh, R.; Manjunatha, U.; Boshoff, H.I.; et al. (November 2008). "PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release". Science. 322 (5906): 1392–1395. Bibcode:2008Sci...322.1392S. doi:10.1126/science.1164571. PMC 2723733
 . PMID 19039139.
. PMID 19039139. - ↑ Ahmad, Z.; Klinkenberg, L.G.; Pinn, M.L.; Fraig, M.M.; Peloquin, C.A.; Bishai, W.R.; Nuermberger, E.L.; Grosset, J.H.; Karakousis, P.C. (2009). "Biphasic Kill Curve of Isoniazid Reveals the Presence of Drug‐Tolerant, Not Drug‐Resistant,Mycobacterium tuberculosis in the Guinea Pig". The Journal of Infectious Diseases. 200 (7): 1136–1143. doi:10.1086/605605. PMID 19686043.
- ↑ Harvey (November 2009). Pharmacology (4th ed.).
- ↑ Jones, David S. (2002). "The Health Care Experiments at Many Farms: The Navajo, Tuberculosis, and the Limits of Modern Medicine, 1952-1962". Bulletin of the History of Medicine. 76 (4): 749–790. doi:10.1353/bhm.2002.0186. PMID 12446978.
- ↑ William Andrew Publishing (2008). Pharmaceutical Manufacturing Encyclopedia (3rd ed.). Norwich, NY: Elsevier Science. pp. 1968–1970. ISBN 9780815515265.
- ↑ Shimizu, Shinkichi; Watanabe, Nanao; Kataoka, Toshiaki; Shoji, Takayuki; Abe, Nobuyuki; Morishita, Sinji; Ichimura, Hisao (2000). "Pyridine and Pyridine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA: 17. doi:10.1002/14356007.a22_399. ISBN 3527306730.
- ↑ Baizer, Manuel M.; Dub, Michael; Gister, Sidney; Steinberg, Nathan G. (1956). "Synthesis of Isoniazid from Citric Acid". Journal of the American Pharmaceutical Association (Scientific ed.). 45 (7): 478–480. doi:10.1002/jps.3030450714. ISSN 0095-9553.
- ↑ "Drugs@FDA". fda.gov. United States Food and Drug Administration. Retrieved 22 August 2016.
- ↑ Smith, L.L.; Foell, Theodore (1959). "Differentiation of Δ4-3-Ketosteroids and Δ1,4-3-Ketosteroids with Isonicotinic Acid Hydrazide". Analytical Chemistry. 31 (1): 102–105. doi:10.1021/ac60145a020.
External links
- Core Curriculum on Tuberculosis (2000) Division of Tuberculosis Elimination, Centers for Disease Control and Prevention
- See Chapter 6, Treatment of LTBI Regimens - Isoniazid::
See Chapter 7 - Treatment of TB Disease Monitoring - Adverse Reactions to First-line TB Drugs - Isoniazid::
See Table 5 First-Line Anti-TB Medications
- Isoniazid Overdose: Recognition and Management American Family Physician 1998 Feb 15