Isatin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1H-indole-2,3-dione | |
| Identifiers | |
| 91-56-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27539 |
| ChEMBL | ChEMBL326294 |
| ChemSpider | 6787 |
| DrugBank | DB02095 |
| ECHA InfoCard | 100.001.889 |
| KEGG | C11129 |
| PubChem | 7054 |
| |
| |
| Properties | |
| C8H5NO2 | |
| Molar mass | 147.1308 g/mol |
| Appearance | Orange-red solid |
| Melting point | 200 °C (392 °F; 473 K) |
| Hazards | |
| EU classification (DSD) |
Harmful (Xn) |
| R-phrases | R22 R36 R37 R38 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Isatin or 1H-indole-2,3-dione is an indole derivative. The compound was first obtained by Erdmann[1] and Laurent[2] in 1841 as a product from the oxidation of indigo dye by nitric acid and chromic acids. The compound is found in many plants, such as Isatis tinctoria, Calanthe discolor and in Couroupita guianensis.[3]
Schiff bases of isatin are investigated for their pharmaceutical properties.[4]
Isatin forms a blue dye (indophenin) if it is mixed with sulfuric acid and crude benzene. The formation of the indophenin was long believed to be a reaction with benzene. Victor Meyer was able to isolate the substance responsible for this reaction from crude benzene. This new heterocyclic compound was thiophene.[5]
Synthesis
The classical methods for the synthesis of isatins are Sandmeyer’s method, the Stolle procedure, and Gassman procedure, all using aniline as substrate.[6]
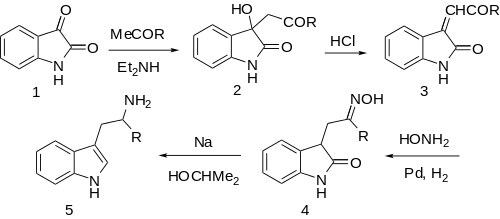
Reactions

See also
References
- ↑ Erdmann, O. L. (1840). "Untersuchungen über den Indigo". Journal für Praktische Chemie. 19 (1): 321–362. doi:10.1002/prac.18400190161.
- ↑ Laurent, A. (1840). "Recherches sur l'indigo". Annales de Chimie et de Physique. 3 (3): 393–434.
- ↑ da Silva, J. F. M.; Garden, S. J.; Pinto, A. C. (2001). "The Chemistry of Isatins: a Review from 1975 to 1999" (pdf). Journal of the Brazilian Chemical Society. 12 (3): 273–324. doi:10.1590/S0103-50532001000300002.
- ↑ Jarrahpour, A. A.; Khalili, D. (2005). "Synthesis of 3,3´-[methylenebis(3,1-phenylenenitrilo)]bis[1,3-dihydro]-2H-indol-2-one as a Novel bis-Schiff Base" (pdf). Molbank. 2005 (4): M437. doi:10.3390/M437.
- ↑ Sumpter, W. C. (1944). "The Chemistry of Isatin". Chemical Reviews. 34 (3): 393–434. doi:10.1021/cr60109a003.
- ↑ Hewawasam, P.; Meanwell, N. A. (1994). "A General Method for the Synthesis of Isatins". Tetrahedron Letters. 35 (40): 7303–7306. doi:10.1016/0040-4039(94)85299-5.
- ↑ S. Pietra, Farmaco, Ed. Sci., 12, 946 (1957).
- ↑ S. Pietra, Farmaco, Ed. Sci., 13, 75 (1958).
- ↑ Pietra, S; Tacconi, G (1958). "Indole derivatives. III. The preparation of alpha-alkyl and of alpha-aryltryptamines". Il Farmaco; edizione scientifica. 13 (12): 893–910. PMID 13619730.
- ↑ Pietra, S; Tacconi, G (1959). "Indole derivatives. Note IV. Preparation of alpha-alkyl- and alpha-aryl-N-methyltryptamines". Il Farmaco; edizione scientifica. 14: 854–66. PMID 13854355.
- ↑ Pietra, S; Tacconi, G (1960). "Indole derivatives. Note 5. Synthesis of alpha-phenyl-beta-methyltryptamine". Il Farmaco; edizione scientifica. 15: 451–67. PMID 13854354.
- ↑ S. Pietra, G. Tacconi, Farmaco, Ed. Sci., 16, 483 (1961).
- ↑ Deligeorgiev, Todor; Vasilev, Aleksey; Vaquero, Juan J.; Alvarez-Builla, Julio (2007). "A green synthesis of isatoic anhydrides from isatins with urea–hydrogen peroxide complex and ultrasound". Ultrasonics Sonochemistry. 14 (5): 497. doi:10.1016/j.ultsonch.2006.12.003. PMID 17258493.
Reviews
- Popp, Prank D. (1975). "Advances in Heterocyclic Chemistry Volume 18". Advances in Heterocyclic Chemistry. 18: 1. doi:10.1016/S0065-2725(08)60127-0. ISBN 9780120206186.
|chapter=ignored (help) - Mesropyan, E. G.; Avetisyan, A. A. (2009). "New isatin derivatives". Russian Journal of Organic Chemistry. 45 (11): 1583. doi:10.1134/S1070428009110013.