Ionic liquids in carbon capture
The use of ionic liquids in carbon capture is a potential application of ionic liquids as absorbents for use in carbon capture and sequestration. Ionic liquids, which are salts that exist as liquids near room temperature, are polar, nonvolatile materials that have been considered for many applications. The urgency of climate change has spurred research into their use in energy-related applications such as carbon capture and storage.
Separations
Carbon capture
Amines are the most prevalent absorbent in postcombustion carbon capture technology today. In particular, monoethanolamine (MEA) has been used in industrial scales in postcombustion carbon capture, as well as in other CO2 separations, such as "sweetening" of natural gas.[1] However, amines are corrosive, degrade over time, and require large industrial facilities. Ionic liquids on the other hand, have low vapor pressures . This property results from their strong Coulombic attractive force. Vapor pressure remains low through the substance's thermal decomposition point (typically >300 °C).[2] In principle, this low vapor pressure simplifies their use and makes them "green" alternatives. Additionally, it reduces risk of contamination of the CO2 gas stream and of leakage into the environment.[3]
The solubility of CO2 in ionic liquids is governed primarily by the anion, less so by the cation.[4] The hexafluorophosphate (PF6–) and tetrafluoroborate (BF4–) anions have been shown to be especially amenable to CO2 capture.[4]
Ionic liquids have been considered as solvents in a variety of liquid-liquid extraction processes.[5] Beside that, ionic liquids have replaced the conventional volatile solvents in industry such as absorption of gases or extractive distillation. Additionally, ionic liquids are used as co-solutes for the generation of aqueous biphasic systems, or purification of biomolecules.
Process
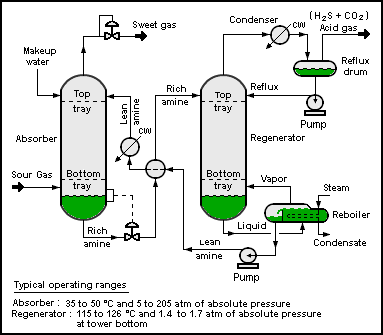
A typical CO2 absorption process consists of a feed gas, an absorption column, a stripper column, and output streams of CO2-rich gas to be sequestered, and CO2-poor gas to be released to the atmosphere. Ionic liquids could follow a similar process to amine gas treating, where the CO2 is regenerated in the stripper using higher temperature. However, ionic liquids can also be stripped using pressure swings or inert gases, reducing the process energy requirement.[3] A current issue with ionic liquids for carbon capture is that they have a lower working capacity than amines. Task-specific ionic liquids which employ chemisorption and physisorption are being developed in an attempt to increase the working capacity. 1-butyl-3-propylamineimidazolium tetrafluoroborate is one example of a TSIL.[2]
Tunability

As required for all separation techniques, ionic liquids exhibit selectivity towards one or more of the phases of a mixture. 1-Butyl-3-methylimidazolium hexafluorophosphate (BMIM-PF6) is a room-temperature ionic liquid that was identified early on as a viable substitute for volatile organic solvents in liquid-liquid separations.[6] Other [PF6]- and [BF4]- containing ionic liquids have been studied for their CO2 absorption properties, as well as 1-ethyl-3-methylimidazolium (EMIM) and unconventional cations like trihexyl(tetradecyl) phosphonium ([P66614]).[3] Selection of different anion and cation combinations in ionic liquids affects their selectivity and physical properties. Additionally, the organic cations in ionic liquids can be "tuned" by changing chain lengths or by substituting radicals.[5] Finally, ionic liquids can be mixed with other ionic liquids, water, or amines to achieve different properties in terms of absorption capacity and heat of absorption. This tunability has led some to call ionic liquids "designer solvents."[7] 1-butyl-3-propylamineimidazolium tetrafluoroborate was specifically developed for CO2 capture; it is designed to employ chemisorption to absorb CO2 and maintain efficiency under repeated absorption/regeneration cycles.[2] Other ionic liquids have been simulated or experimentally tested for potential use as CO2 absorbents.
Industrial applications
Industrial operations require an energy efficient and environmentally friendly process for CO2 capture. Currently, CO2 capture uses mostly amine-based absorption technologies, which are energy intensive and solvent intensive. Volatile organic compounds alone in chemical processes represent a multi-billion dollar industry.[6] Therefore, ionic liquids offer an alternative that requires less energy. Due to the properties of ionic liquids, they have potential for large-scale implementation in post-combustion CO2 capture.
During the capture process, the anion and cation play a crucial role in the dissolution of CO2. Spectroscopic results suggest a favorable interaction between the anion and CO2, wherein CO2 molecules preferentially attach to the anion. Furthermore, intermolecular forces, such as hydrogen bonds, van der Waals bonds, and electrostatic attraction, contributes to the solubility of CO2 in ionic liquids. This makes ionic liquids promising candidates for CO2 capture because the solubility of CO2 can be modeled accurately by the regular solubility theory (RST), which reduces operational costs in developing more sophisticated model to monitor the capture process.
For example, due to their practical properties, ionic liquids have been shifting more from academic labs into industrial applications. Ionic liquids have been marketed as Gasguard Subatmospheric System by Air Products. This application was specifically for gas absorption, where it has proven to be twice as effective in performance as normal absorption techniques.
One of the main drawbacks of ionic liquids is their high viscosity, which complicates their use in industrial operations. Supported ionic liquid phases (SILPs) are one proposed solution to this problem.[5]
References
- ↑ Arthur Kohl and Richard Nielson (1997). Gas Purification (5th ed.). Gulf Publishing. ISBN 0-88415-220-0.
- 1 2 3 Bates, Eleanor D.; Mayton, Rebecca D.; Ntai, Ioanna; Davis, James H. (2002). "CO2 Capture by a Task-Specific Ionic Liquid". Journal of the American Chemical Society. 124 (6): 926–927. doi:10.1021/ja017593d. ISSN 0002-7863.
- 1 2 3 Zhang, Xiangping; Zhang, Xiaochun; Dong, Haifeng; Zhao, Zhijun; Zhang, Suojiang; Huang, Ying (2012). "Carbon capture with ionic liquids: overview and progress". Energy & Environmental Science. 5 (5): 6668. doi:10.1039/c2ee21152a. ISSN 1754-5692.
- 1 2 Ramdin, Mahinder; de Loos, Theo W.; Vlugt, Thijs J.H. (2012). "State-of-the-Art of CO2 Capture with Ionic Liquids". Industrial & Engineering Chemistry Research. 51 (24): 8149–8177. doi:10.1021/ie3003705. ISSN 0888-5885.
- 1 2 3 Rodríguez, Héctor (2016). "Ionic Liquids for Better Separation Processes". doi:10.1007/978-3-662-48520-0. ISSN 2196-6982.
- 1 2 Huddleston, Jonathan G.; Willauer, Heather D.; Swatloski, Richard P.; Visser, Ann E.; Rogers, Robin D. (1998). "Room temperature ionic liquids as novel media for 'clean' liquid–liquid extraction". Chem. Commun. (16): 1765–1766. doi:10.1039/A803999B. ISSN 1359-7345.
- ↑ Freemantle, Michael (1998). "Designer Solvents". Chemical & Engineering News. 76 (13): 32–37. doi:10.1021/cen-v076n013.p032. ISSN 0009-2347.
Further reading
- Blanchard, Lynnette A.; Hancu, Dan; Beckman, Eric J.; Brennecke, Joan F. (1999). "Green processing using ionic liquids and CO2". Nature. 399 (6731): 28–29. doi:10.1038/19887. ISSN 0028-0836.
- Camper, Dean; Bara, Jason E.; Gin, Douglas L.; Noble, Richard D. (2008). "Room-Temperature Ionic Liquid−Amine Solutions: Tunable Solvents for Efficient and Reversible Capture of CO2". Industrial & Engineering Chemistry Research. 47 (21): 8496–8498. doi:10.1021/ie801002m. ISSN 0888-5885.