Ion-exchange resin

An ion-exchange resin or ion-exchange polymer is a resin or polymer that acts as a medium for ion exchange. It is an insoluble matrix (or support structure) normally in the form of small (0.5–1 mm diameter) microbeads, usually white or yellowish, fabricated from an organic polymer substrate. The beads are typically porous, providing a large surface area on and inside them. The trapping of ions occurs along with the accompanying release of other ions, and thus the process is called ion exchange. There are multiple types of ion-exchange resin. Most commercial resins are made of polystyrene sulfonate.[1]
Ion-exchange resins are widely used in different separation, purification, and decontamination processes. The most common examples are water softening and water purification. In many cases ion-exchange resins were introduced in such processes as a more flexible alternative to the use of natural or artificial zeolites. Also, ion-exchange resins are highly effective in the biodiesel filtration process.
Types of resins
Most typical ion-exchange resins are based on crosslinked polystyrene. The actual ion-exchanging sites are introduced after polymerisation. Additionally, in the case of polystyrene, crosslinking is introduced by copolymerisation of styrene and a few percent of divinylbenzene (non-crosslinked polymers are soluble in water). Crosslinking decreases ion-exchange capacity of the resin and prolongs the time needed to accomplish the ion-exchange processes but improves the robustness of the resin. Particle size also influences the resin parameters; smaller particles have larger outer surface, but cause larger head loss in the column processes.[2]
Besides being made as bead-shaped materials, ion-exchange resins are also produced as membranes. These ion-exchange membranes, which are made of highly cross-linked ion-exchange resins that allow passage of ions, but not of water, are used for electrodialysis.
Four main types of ion-exchange resins differ in their functional groups:
- strongly acidic, typically featuring sulfonic acid groups, e.g. sodium polystyrene sulfonate or polyAMPS,
- strongly basic, typically featuring quaternary amino groups, for example, trimethylammonium groups, e.g. polyAPTAC),
- weakly acidic, typically featuring carboxylic acid groups,
- weakly basic, typically featuring primary, secondary, and/or tertiary amino groups, e.g. polyethylene amine.
Specialised ion-exchange resins are also known such as chelating resins (iminodiacetic acid, thiourea-based resins, and many others).
Anion resins and cation resins are the two most common resins used in the ion-exchange process. While anion resins attract negatively charged ions, cation resins attract positively charged ions.
Anion resins
Anion resins may be either strongly or weakly basic. Strongly basic anion resins maintain their positive charge across a wide pH range, whereas weakly basic anion resins are neutralized at higher pH levels.[3] Weakly basic resins do not maintain their charge at a high pH because they undergo deprotonation.[3] They do, however, offer excellent mechanical and chemical stability. This, combined with a high rate of ion exchange, make weakly base anion resins well suited for the organic salts.
For anion resins, regeneration typically involves treatment of the resin with a strongly basic solution, e.g. aqueous sodium hydroxide. During regeneration, the regenerant chemical is passed through the resin, and trapped negative ions are flushed out, renewing the resin exchange capacity.
Cation-exchange resin
Formula: R−H
Reaction: RH+ + C+ !RC+ + H+
Anion-exchange resin
Formula: N(R)4−OH
Reaction:
- N(R)4−OH + HCl = N(R)4Cl + H2O
Anion-exchange chromatography makes use of this principle to extract and purify materials from mixtures or solutions.
Uses
Water softening
In this application, ion-exchange resins are used to replace the magnesium and calcium ions found in hard water with sodium ions. When the resin is fresh, it contains sodium ions at its active sites. When in contact with a solution containing magnesium and calcium ions (but a low concentration of sodium ions), the magnesium and calcium ions preferentially migrate out of solution to the active sites on the resin, being replaced in solution by sodium ions. This process reaches equilibrium with a much lower concentration of magnesium and calcium ions in solution than was started with.
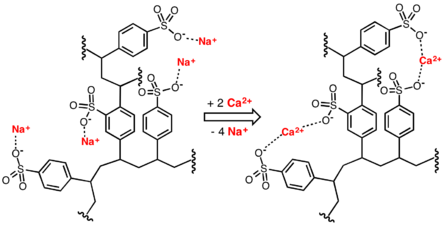
The resin can be recharged by washing it with a solution containing a high concentration of sodium ions (e.g. it has large amounts of common salt (NaCl) dissolved in it). The calcium and magnesium ions migrate off the resin, being replaced by sodium ions from the solution until a new equilibrium is reached. The salt is used to recharge an ion-exchange resin which itself is used to soften the water.
Water purification
In this application, ion-exchange resins are used to remove poisonous (e.g. copper) and heavy metal (e.g. lead or cadmium) ions from solution, replacing them with more innocuous ions, such as sodium and potassium.
Few ion-exchange resins remove chlorine or organic contaminants from water – this is usually done by using an activated charcoal filter mixed in with the resin. There are some ion-exchange resins that do remove organic ions, such as MIEX (magnetic ion-exchange) resins. Domestic water purification resin is not usually recharged – the resin is discarded when it can no longer be used.
Production of high-purity water
Water of highest purity is required for electronics, scientific experiments, production of superconductors, and nuclear industry, among others. Such water is produced using ion-exchange processes or combinations of membrane and ion-exchange methods. Cations are replaced with hydrogen ions using cation-exchange resins; anions are replaced with hydroxyls using anion-exchange resins. The hydrogen ions and hydroxyls recombine producing water molecules. Thus, no ions remain in the produced water. The purification process is usually performed in several steps with "mixed bed ion-exchange columns" at the end of the technological chain.
Ion-exchange in metal separation

Ion-exchange processes are used to separate and purify metals, including separating uranium from plutonium and other actinides, including thorium; and lanthanum, neodymium, ytterbium, samarium, lutetium, from each other and the other lanthanides. There are two series of rare earth metals, the lanthanides and the actinides. Members of each family have very similar chemical and physical properties. Ion-exchange was for many years the only practical way to separate the rare earths in large quantities. This application was developed in the 1940s by Frank Spedding. Subsequently, solvent extraction has mostly supplanted use of ion exchange resins except for the highest purity products.
A very important case is the PUREX process (plutonium-uranium extraction process) which is used to separate the plutonium and the uranium from the spent fuel products from a nuclear reactor, and to be able to dispose of the waste products. Then, the plutonium and uranium are available for making nuclear-energy materials, such as new reactor fuel and nuclear weapons.
Ion-exchange beads are also an essential component in In-situ leach uranium mining. In-situ recovery involves the extraction of uranium-bearing water (grading as low as .05% U3O8) through boreholes. The extracted uranium solution is then filtered through the resin beads. Through an ion exchange process, the resin beads attract uranium from the solution. Uranium loaded resins are then transported to a processing plant, where U3O8 is separated from the resin beads and yellowcake is produced. The resin beads can then be returned to the ion exchange facility where they are reused.
The ion-exchange process is also used to separate other sets of very similar chemical elements, such as zirconium and hafnium, which incidentally is also very important for the nuclear industry. Zirconium is practically transparent to free neutrons, used in building reactors, but hafnium is a very strong absorber of neutrons, used in reactor control rods.
Catalysis
In chemistry ion-exchange resins in the acid form are known to catalyze organic reactions. See for instance self-condensation.
Juice purification
Ion-exchange resins are used in the manufacture of fruit juices such as orange and cranberry juice, where they are used to remove bitter-tasting components and so improve the flavor. This allows tart or poorer-tasting fruit sources to be used for juice production.
Sugar manufacturing
Ion-exchange resins are used in the manufacturing of sugar from various sources. They are used to help convert one type of sugar into another type of sugar, and to decolorize and purify sugar syrups.
Pharmaceuticals
Ion-exchange resins are used in the manufacturing of pharmaceuticals, not only for catalyzing certain reactions but also for isolating and purifying pharmaceutical active ingredients. Three ion-exchange resins, sodium polystyrene sulfonate, colestipol, and cholestyramine, are used as active ingredients. Sodium polystyrene sulfonate is a strongly acidic ion-exchange resin and is used to treat hyperkalemia. Colestipol is a weakly basic ion-exchange resin and is used to treat hypercholesterolemia. Cholestyramine is a strongly basic ion-exchange resin and is also used to treat hypercholesterolemia. Colestipol and cholestyramine are known as bile acid sequestrants.
Ion-exchange resins are also used as excipients in pharmaceutical formulations such as tablets, capsules, gums, and suspensions. In these uses the ion-exchange resin can have several different functions, including taste-masking, extended release, tablet disintegration, increased bioavailability, and improving the chemical stability of the active ingredients.
Notes
- ↑ François Dardel and Thomas V. Arden "Ion Exchangers" in Ullmann's Encyclopedia of Industrial Chemistry, 2008, Wiley-VCH, Weinheim. doi:10.1002/14356007.a14_393.pub2.
- ↑ IUPAC "strongly discourages" the use of the term "ion-exchange resin" to refer to an ion-exchange polymer, but the usage remains common: International Union of Pure and Applied Chemistry (2004), "Definitions of Terms Relating to Reactions of Polymers and to Functional Polymeric Materials (IUPAC Recommendations 2003)" (PDF), Pure Appl. Chem., 76 (4): 889–906, doi:10.1351/pac200476040889
- 1 2 Wikibooks:Proteomics/Protein Separations - Chromatography/Ion exchange#Anion Exchangers.
Additional reading
- "Ion Exchange Chemistry and Operation". Remco Engineering. Retrieved 2014-05-16.
- Friedrich G. Helfferich (1962). Ion Exchange. Courier Dover Publications. ISBN 978-0-486-68784-1.
- Ion Exchangers (K. Dorfner, ed.), Walter de Gruyter, Berlin, 1991.
- C. E. Harland, Ion exchange: Theory and Practice, The Royal Society of Chemistry, Cambridge, 1994.
- Ion exchange (D. Muraviev, V. Gorshkov, A. Warshawsky), M. Dekker, New York, 2000.
- A. A. Zagorodni, Ion Exchange Materials: Properties and Applications, Elsevier, Amsterdam, 2006.
- What is Deionized Water?