Interleukin 10 receptor, alpha subunit
| IL10RA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||
| |||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | IL10RA, CD210, CD210a, CDW210A, HIL-10R, IL-10R1, IL10R, Interleukin 10 receptor, alpha subunit, interleukin 10 receptor subunit alpha | ||||||||||||||||
| External IDs | MGI: 96538 HomoloGene: 1196 GeneCards: IL10RA | ||||||||||||||||
| |||||||||||||||||
| RNA expression pattern | |||||||||||||||||
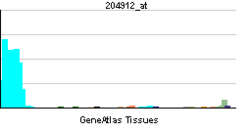 | |||||||||||||||||
| More reference expression data | |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 11: 117.99 – 118 Mb | Chr 9: 45.25 – 45.27 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
Interleukin 10 receptor, alpha subunit is a subunit for the interleukin-10 receptor. IL10RA, is its human gene.
IL10RA has also recently been designated CDW210A (cluster of differentiation W210A).
Function
The protein encoded by this gene is a receptor for interleukin 10. This protein is structurally related to interferon receptors. It has been shown to mediate the immunosuppressive signal of interleukin 10, and thus inhibits the synthesis of proinflammatory cytokines. This receptor is reported to promote survival of progenitor myeloid cells through the insulin receptor substrate-2/PI 3-kinase/AKT pathway. Activation of this receptor leads to tyrosine phosphorylation of JAK1 and TYK2 kinases.[3]
Interactions
Interleukin 10 receptor, alpha subunit has been shown to interact with:
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ "Entrez Gene: IL10RA interleukin 10 receptor, alpha".
- ↑ Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW (December 1993). "A receptor for interleukin 10 is related to interferon receptors". Proc. Natl. Acad. Sci. U.S.A. 90 (23): 11267–71. doi:10.1073/pnas.90.23.11267. PMC 47963
 . PMID 8248239.
. PMID 8248239. - ↑ Josephson K, Logsdon NJ, Walter MR (July 2001). "Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site". Immunity. 15 (1): 35–46. doi:10.1016/s1074-7613(01)00169-8. PMID 11485736.
- ↑ Tan JC, Braun S, Rong H, DiGiacomo R, Dolphin E, Baldwin S, Narula SK, Zavodny PJ, Chou CC (May 1995). "Characterization of recombinant extracellular domain of human interleukin-10 receptor". J. Biol. Chem. 270 (21): 12906–11. doi:10.1074/jbc.270.21.12906. PMID 7759550.
- ↑ Josephson K, McPherson DT, Walter MR (December 2001). "Purification, crystallization and preliminary X-ray diffraction of a complex between IL-10 and soluble IL-10R1". Acta Crystallogr. D. 57 (Pt 12): 1908–11. doi:10.1107/s0907444901016249. PMID 11717514.
- ↑ Hoover DM, Schalk-Hihi C, Chou CC, Menon S, Wlodawer A, Zdanov A (May 1999). "Purification of receptor complexes of interleukin-10 stoichiometry and the importance of deglycosylation in their crystallization". Eur. J. Biochem. 262 (1): 134–41. doi:10.1046/j.1432-1327.1999.00363.x. PMID 10231374.
- ↑ Usacheva A, Sandoval R, Domanski P, Kotenko SV, Nelms K, Goldsmith MA, Colamonici OR (December 2002). "Contribution of the Box 1 and Box 2 motifs of cytokine receptors to Jak1 association and activation". J. Biol. Chem. 277 (50): 48220–6. doi:10.1074/jbc.M205757200. PMID 12374810.
- ↑ Usacheva A, Kotenko S, Witte MM, Colamonici OR (August 2002). "Two distinct domains within the N-terminal region of Janus kinase 1 interact with cytokine receptors". J. Immunol. 169 (3): 1302–8. doi:10.4049/jimmunol.169.3.1302. PMID 12133952.
Further reading
- Donnelly RP, Dickensheets H, Finbloom DS (1999). "The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes". J. Interferon Cytokine Res. 19 (6): 563–73. doi:10.1089/107999099313695. PMID 10433356.
- Carson WE, Lindemann MJ, Baiocchi R, Linett M, Tan JC, Chou CC, Narula S, Caligiuri MA (1995). "The functional characterization of interleukin-10 receptor expression on human natural killer cells". Blood. 85 (12): 3577–85. PMID 7540068.
- Ho AS, Wei SH, Mui AL, Miyajima A, Moore KW (1995). "Functional regions of the mouse interleukin-10 receptor cytoplasmic domain". Mol. Cell. Biol. 15 (9): 5043–53. PMC 230751
 . PMID 7544437.
. PMID 7544437. - Tan JC, Braun S, Rong H, DiGiacomo R, Dolphin E, Baldwin S, Narula SK, Zavodny PJ, Chou CC (1995). "Characterization of recombinant extracellular domain of human interleukin-10 receptor". J. Biol. Chem. 270 (21): 12906–11. doi:10.1074/jbc.270.21.12906. PMID 7759550.
- Taniyama T, Takai S, Miyazaki E, Fukumura R, Sato J, Kobayashi Y, Hirakawa T, Moore KW, Yamada K (1995). "The human interleukin-10 receptor gene maps to chromosome 11q23.3". Hum. Genet. 95 (1): 99–101. doi:10.1007/BF00225083. PMID 7814035.
- Liu Y, Wei SH, Ho AS, de Waal Malefyt R, Moore KW (1994). "Expression cloning and characterization of a human IL-10 receptor". J. Immunol. 152 (4): 1821–9. PMID 8120391.
- Ho AS, Liu Y, Khan TA, Hsu DH, Bazan JF, Moore KW (1994). "A receptor for interleukin 10 is related to interferon receptors". Proc. Natl. Acad. Sci. U.S.A. 90 (23): 11267–71. doi:10.1073/pnas.90.23.11267. PMC 47963
 . PMID 8248239.
. PMID 8248239. - Tan JC, Indelicato SR, Narula SK, Zavodny PJ, Chou CC (1993). "Characterization of interleukin-10 receptors on human and mouse cells". J. Biol. Chem. 268 (28): 21053–9. PMID 8407942.
- Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS, Carson WE, Kordula T, Caligiuri MA, Hawley RG, Fey GH, Baumann H (1996). "Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements". J. Biol. Chem. 271 (24): 13968–75. doi:10.1074/jbc.271.24.13968. PMID 8662928.
- Zdanov A, Schalk-Hihi C, Wlodawer A (1997). "Crystal structure of human interleukin-10 at 1.6 A resolution and a model of a complex with its soluble receptor". Protein Sci. 5 (10): 1955–62. doi:10.1002/pro.5560051001. PMC 2143256
 . PMID 8897595.
. PMID 8897595. - Kotenko SV, Krause CD, Izotova LS, Pollack BP, Wu W, Pestka S (1997). "Identification and functional characterization of a second chain of the interleukin-10 receptor complex". EMBO J. 16 (19): 5894–903. doi:10.1093/emboj/16.19.5894. PMC 1170220
 . PMID 9312047.
. PMID 9312047. - Hoover DM, Schalk-Hihi C, Chou CC, Menon S, Wlodawer A, Zdanov A (1999). "Purification of receptor complexes of interleukin-10 stoichiometry and the importance of deglycosylation in their crystallization". Eur. J. Biochem. 262 (1): 134–41. doi:10.1046/j.1432-1327.1999.00363.x. PMID 10231374.
- Müschen A, Mirmohammadsadegh A, Jarzebska-Deussen B, Abts HF, Ruzicka T, Michel G (1999). "Differential IL-10 receptor gene expression in acute versus chronic atopic eczema. Modulation by immunosuppressive drugs and cytokines in normal cultured keratinocytes". Inflamm. Res. 48 (10): 539–43. doi:10.1007/s000110050500. PMID 10563471.
- Corinti S, Albanesi C, la Sala A, Pastore S, Girolomoni G (2001). "Regulatory activity of autocrine IL-10 on dendritic cell functions". J. Immunol. 166 (7): 4312–8. doi:10.4049/jimmunol.166.7.4312. PMID 11254683.
- Josephson K, Logsdon NJ, Walter MR (2001). "Crystal structure of the IL-10/IL-10R1 complex reveals a shared receptor binding site". Immunity. 15 (1): 35–46. doi:10.1016/S1074-7613(01)00169-8. PMID 11485736.
- Crepaldi L, Gasperini S, Lapinet JA, Calzetti F, Pinardi C, Liu Y, Zurawski S, de Waal Malefyt R, Moore KW, Cassatella MA (2001). "Up-regulation of IL-10R1 expression is required to render human neutrophils fully responsive to IL-10". J. Immunol. 167 (4): 2312–22. doi:10.4049/jimmunol.167.4.2312. PMID 11490020.
- Zhou JH, Broussard SR, Strle K, Freund GG, Johnson RW, Dantzer R, Kelley KW (2001). "IL-10 inhibits apoptosis of promyeloid cells by activating insulin receptor substrate-2 and phosphatidylinositol 3'-kinase". J. Immunol. 167 (8): 4436–42. doi:10.4049/jimmunol.167.8.4436. PMID 11591769.
- Josephson K, McPherson DT, Walter MR (2002). "Purification, crystallization and preliminary X-ray diffraction of a complex between IL-10 and soluble IL-10R1". Acta Crystallogr. D. 57 (Pt 12): 1908–11. doi:10.1107/S0907444901016249. PMID 11717514.
- Wolk K, Kunz S, Asadullah K, Sabat R (2002). "Cutting edge: immune cells as sources and targets of the IL-10 family members?". J. Immunol. 168 (11): 5397–402. doi:10.4049/jimmunol.168.11.5397. PMID 12023331.
This article incorporates text from the United States National Library of Medicine, which is in the public domain.