Tuberculosis diagnosis
Tuberculosis is diagnosed by finding Mycobacterium tuberculosis bacteria in a clinical specimen taken from the patient. While other investigations may strongly suggest tuberculosis as the diagnosis, they cannot confirm it.
A complete medical evaluation for tuberculosis (TB) must include a medical history, a physical examination, a chest X-ray and microbiological examination (of sputum or some other appropriate sample). It may also include a tuberculin skin test, other scans and X-rays, surgical biopsy.
Medical history
The medical history includes obtaining the symptoms of pulmonary TB: productive, prolonged cough of three or more weeks, chest pain, and hemoptysis. Systemic symptoms include low grade remittent fever, chills, night sweats, appetite loss, weight loss, easy fatiguability, and production of sputum that starts out mucoid but changes to purulent.[1] Other parts of the medical history include prior TB exposure, infection or disease and medical conditions that increase risk for TB disease such as HIV infection. Depending on the sort of patient population surveyed, as few as 20%, or as many as 75% of pulmonary tuberculosis cases may be without symptoms.[2]
Tuberculosis should be suspected when a pneumonia-like illness has persisted longer than three weeks, or when a respiratory illness in an otherwise healthy individual does not respond to regular antibiotics.
Physical examination
A physical examination is done to assess the patient's general health. It cannot be used to confirm or rule out TB. However, certain findings are suggestive of TB. For example, blood in the sputum, significant weight loss and drenching night sweats may be due to TB.
Microbiological studies
 Distinctive clusters of colorless Mycobacterium tuberculosis form in this culture. | |
| Gram | + |
|---|---|
| Shape | rods |
A definitive diagnosis of tuberculosis can only be made by culturing Mycobacterium tuberculosis organisms from a specimen taken from the patient (most often sputum, but may also include pus, CSF, biopsied tissue, etc.).[1] A diagnosis made other than by culture may only be classified as "probable" or "presumed". For a diagnosis negating the possibility of tuberculosis infection, most protocols require that two separate cultures both test negative.[1]
Sputum
Sputum smears and cultures should be done for acid-fast bacilli if the patient is producing sputum.[1] The preferred method for this is fluorescence microscopy (auramine-rhodamine staining), which is more sensitive than conventional Ziehl-Neelsen staining.[3] In cases where there is no spontaneous sputum production, a sample can be induced, usually by nebulized inhalation of a saline or saline with bronchodilator solution. A comparative study found that inducing three sputum samples is more sensitive than three gastric washings.[4]
Alternative sampling
In patients incapable of producing a sputum sample, common alternative sample sources for diagnosing pulmonary tuberculosis include gastric washings, laryngeal swab, bronchoscopy (with bronchoalveolar lavage, bronchial washings, and/or transbronchial biopsy), and fine needle aspiration (transtracheal or transbronchial). In some cases, a more invasive technique is necessary, including tissue biopsy during mediastinoscopy or thoracoscopy.
PCR
Other mycobacteria are also acid-fast. If the smear is positive, PCR or gene probe tests can distinguish M. tuberculosis from other mycobacteria. Even if sputum smear is negative, tuberculosis must be considered and is only excluded after negative cultures.
Other
Many types of cultures are available.[5] Traditionally, cultures have used the Löwenstein-Jensen (LJ), Kirchner, or Middlebrook media (7H9, 7H10, and 7H11). A culture of the AFB can distinguish the various forms of mycobacteria, although results from this may take four to eight weeks for a conclusive answer. New automated systems that are faster include the MB/BacT, BACTEC 9000, VersaTREK, and the Mycobacterial Growth Indicator Tube (MGIT). The Microscopic Observation Drug Susceptibility assay culture may be a faster and more accurate method.[6]
Radiography
Chest X-ray and CT
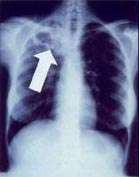
In active pulmonary TB, infiltrates or consolidations and/or cavities are often seen in the upper lungs with or without mediastinal or hilar lymphadenopathy or pleural effusions ( tuberculous pleurisy). However, lesions may appear anywhere in the lungs. In disseminated TB a pattern of many tiny nodules throughout the lung fields is common - the so-called miliary TB. In HIV and other immunosuppressed persons, any abnormality may indicate TB or the chest X-ray may even appear entirely normal.
Abnormalities on chest radiographs may be suggestive of, but are not necessarily diagnostic of, TB. However, chest radiographs may be used to rule out the possibility of pulmonary TB in a person who has a positive reaction to the tuberculin skin test and no symptoms of the disease.
Cavitation or consolidation of the apexes of the upper lobes of the lung or the tree-in-bud sign[7] may be visible on an affected patient's chest X-ray.[1] The tree-in-bud sign may appear on the chest CTs of some patients affected by tuberculosis, but it is not specific to tuberculosis.[7]
Abreugraphy
A variant of the chest X-Ray, abreugraphy (from the name of its inventor, Dr. Manuel Dias de Abreu) was a small radiographic image, also called miniature mass radiography (MMR) or miniature chest radiograph. Though its resolution is limited (it doesn't allow the diagnosis of lung cancer, for example) it is sufficiently accurate for diagnosis of tuberculosis.
Much less expensive than traditional X-Ray, MMR was quickly adopted and extensively utilized in some countries, in the 1950s. For example, in Brazil and in Japan, tuberculosis prevention laws went into effect, obligating ca. 60% of the population to undergo MMR screening.
The procedure went out of favor, as the incidence of tuberculosis dramatically decreased, but is still used in certain situations, such as the screening of prisoners and immigration applicants..
Immunological test
ALS Assay
Antibodies from Lymphocyte Secretion or Antibody in Lymphocyte Supernatant or ALS Assay is an immunological assay to detect active diseases like tuberculosis, cholera, typhoid etc. Recently, ALS assay nods the scientific community as it is rapidly used for diagnosis of tuberculosis. The principle is based on the secretion of antibody from in vivo activated plasma B cells found in blood circulation for a short period of time in response to TB-antigens during active TB infection rather than latent TB infection.
Transdermal Patch
A similar approach to the ALS assay. The transdermal patch is a suggested method of detecting active M.tuberculosis circulating within blood vessels of patient. This skin patch contains antibodies recognizing the secreted bacterial protein MPB-64 passing through the blood capillaries of the skin creating an immunological response.[8] If the patch detects this secreted bacterial protein, the surrounding skin will redden.[8]
Tuberculin skin test
Two tests are available: the Mantoux and Heaf tests.
Mantoux skin test

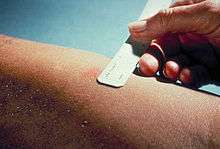
The Mantoux skin test is used in the United States and is endorsed by the American Thoracic Society and Centers for Disease Control and Prevention (CDC).
If a person has had a history of a positive tuberculin skin test, another skin test is not needed.
Heaf test
The Heaf test was used in the United Kingdom until 2005, and is graded on a four-point scale. The Mantoux test is now used.
The equivalent Mantoux test positive levels done with 10 TU (0.1 ml 100 TU/ml, 1:1000) are
- 0–4 mm induration (Heaf 0 to 1)
- 5–14 mm induration (Heaf 2)
- Greater than 15 mm induration (Heaf 3 to 5)
CDC classification of tuberculin reaction
An induration (palpable raised hardened area of skin) of more than 5–15 mm (depending upon the person's risk factors) to 10 Mantoux units is considered a positive result, indicating TB infection.
- 5 mm or more is positive in
- HIV-positive person
- Recent contacts of TB case
- Persons with nodular or fibrotic changes on CXR consistent with old healed TB
- Patients with organ transplants and other immunosuppressed patients
- 10 mm or more is positive in
- Recent arrivals (less than 5 years) from high-prevalent countries
- Injection drug users
- Residents and employees of high-risk congregate settings (e.g., prisons, nursing homes, hospitals, homeless shelters, etc.)
- Mycobacteriology lab personnel
- Persons with clinical conditions that place them at high risk (e.g., diabetes, prolonged corticosteroid therapy, leukemia, end-stage renal disease, chronic malabsorption syndromes, low body weight, etc.)
- Children less than 4 years of age, or children and adolescents exposed to adults in high-risk categories
- 15 mm or more is positive in
- Persons with no known risk factors for TB
- (Note: Targeted skin testing programs should only be conducted among high-risk groups)
A tuberculin test conversion is defined as an increase of 10 mm or more within a 2-year period, regardless of age.
BCG vaccine and tuberculin skin test
There is disagreement on the use of the Mantoux test on people who have been immunized with BCG. The US recommendation is that in administering and interpreting the Mantoux test, previous BCG vaccination should be ignored; the UK recommendation is that interferon-γ tests should be used to help interpret positive tuberculin tests, also, the UK does not recommend serial tuberculin skin testing in people who have had BCG (a key part of the US strategy). In their guidelines on the use of QuantiFERON Gold the US Centers for Disease Control and Prevention state that whereas Quantiferon Gold is not affected by BCG inoculation tuberculin tests can be affected.[9] In general the US approach is likely to result in more false positives and more unnecessary treatment with potentially toxic drugs; the UK approach is as sensitive in theory and should also be more specific, because of the use of interferon-γ tests.
Under the US recommendations, diagnosis and treatment of latent tuberculosis infection (LTBI) is considered for any BCG-vaccinated person whose skin test is 10 mm or greater, if any of these circumstances are present:
- Was in contact with another person with infectious TB
- Was born or has resided in a high TB prevalence country
- Is continually exposed to populations where TB prevalence is high.
ve been reviewed in detail.[5][10]
Adenosine deaminase
In 2007, a systematic review of adenosine deaminase by the NHS Health Technology Assessment Programme concluded "There is no evidence to support the use of ADA tests for the diagnosis of pulmonary TB. However, there is considerable evidence to support their use in pleural fluid samples for diagnosis of pleural TB, where sensitivity was very high, and to a slightly lesser extent for TB meningitis. In both pleural TB and TB meningitis, ADA tests had higher sensitivity than any other tests."[10]
Nucleic acid amplification tests (NAAT)
This is a heterogeneous group of tests that use either the polymerase chain reaction (PCR) technique or Transcription mediated amplification (TMA) or other forms of nucleic acid amplification methods to detect mycobacterial nucleic acid. These test vary in which nucleic acid sequence they detect and vary in their accuracy. The two most common commercially available tests are the amplified mycobacterium tuberculosis direct test (MTD, Gen-Probe) and Amplicor (Roche Diagnostics). In 2007, a systematic review of NAAT by the NHS Health Technology Assessment Programme concluded that "NAAT test accuracy to be far superior when applied to respiratory samples as opposed to other specimens. Although the results were not statistically significant, the AMTD test appears to perform better than other currently available commercial tests."[10]
In a more recent before-after observational study, found that use of the MTD test reduce inappropriate tuberculosis therapy. The study found the accuracy of the MTD test as follows:[11]
Overall
- sensitivity 92%
- specificity 99%
Smear positive patients
- sensitivity 99%
- specificity 98%
Smear negative patients
- sensitivity 62%
- specificity 99%
Full blood count
Although a full blood count is never diagnostic, normocytic anemia and lymphopenia are common. Neutrophilia is rarely found [iron deficiency anemia may develop with isoniazid treatment]. Urea and electrolytes are usually normal, although hypocalcemia and hyponatremia are possible in tuberculous meningoencephalitis due to SIADH. In advanced disease, hypoalbuminemia, hyperproteinemia, and hyperglobulinemia may be present.[12]
Erythrocyte sedimentation rate is usually raised.
Interferon-γ release assays
Interferon-γ (interferon-gamma) release assays (IGRAs) are new developments in TB infection testing. IGRAs are based on the ability of the Mycobacterium tuberculosis antigens for early secretory antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) to stimulate host production of interferon-gamma. Because these antigens are not present in non-tuberculous mycobacteria or in any BCG vaccine variant, these tests can distinguish latent tuberculosis infection (LTBI).
The blood tests QuantiFERON-TB Gold In-Tube and T-SPOT.TB use these antigens to detect people with tuberculosis. Lymphocytes from the patient's blood are incubated with the antigens. These tests are called interferon γ tests and are not equivalent.[13] If the patient has been exposed to tuberculosis before, T lymphocytes produce interferon γ in response. The QuantiFERON-TB Gold In-Tube uses an ELISA format to detect the whole blood production of interferon γ with great sensitivity (89%). The distinction between the tests is that QuantiFERON-TB Gold quantifies the total amount of interferon γ when whole blood is exposed to the antigens(ESAT-6,CFP-10 and TB 7.7(p4)), whereas Guidelines for the use of the FDA approved QuantiFERON-TB Gold were released by the CDC in December 2005. In October 2007, the FDA gave approval of QuantiFERON-TB Gold In Tube for use in the United States.
The enzyme-linked immunospot assay (ELISPOT) is another blood test available in the UK that may replace the skin test for diagnosis.[14][15][16] T-SPOT.TB,[17] a type of ELISPOT assay,[18] counts the number of activated T lymphocytes that secrete interferon γ.
For diagnosing latent TB, three systematic reviews of IGRAs concluded the tests noted excellent specificity for the tests to distinguish latent TB from prior vaccination.[10][19]
According to a study from Korea, where there is a high prevalence of LTBI, QuantiFERON-TB Gold and T-SPOT.TB have good sensitivity but reduced specificity for diagnosing active TB, due to their ability to detect latent TB.[20] In a recently published metaanalysis,[21] with data from both developed and developing countries, QuantiFERON-TB Gold In Tube had a pooled sensitivity for active TB of 81% and specificity of 99.2%, whereas T-SPOT.TB had a pooled sensitivity of 87.5% and specificity of 86.3%. In head-to-head comparisons, the sensitivity of IGRAs surpassed TST. The authors concluded that IGRAs are "superior to the TST for detecting confirmed active TB disease..."
A study at Stanford University conform that addition of immune boosters can make the IGRA more reliable in terms of separating positive from negative individuals.[22] A study from the University of Southampton shows that variations in environmental temperatures can have a profound effect on the performance of IGRA.
Tuberculosis classification system used in the US
The current clinical classification system for TB (Class 0 to 5) is based on the pathogenesis of the disease.
The U.S. Citizenship and Immigration Services has an additional TB classification (Class A, B1, or B2) for immigrants and refugees developed by the Centers for Disease Control and Prevention (CDC). The (Class) B notification program is an important screening strategy to identify new arrivals who have a high risk for TB.
References
- 1 2 3 4 5 Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson; & Mitchell, Richard N. (2007). Robbins Basic Pathology (8th ed.). Saunders Elsevier. pp. 516-522 ISBN 978-1-4160-2973-1
- ↑ Burke and Parnell. Minimal Pulmonary Tuberculosis. 1948. 59:348 Canadian Medical Association Journal.
- ↑ Steingart K, Henry M, Ng V, et al. (2006). "Fluorescence versus conventional sputum smear microscopy for tuberculosis: a systematic review". Lancet Infect Dis. 6 (9): 570–81. doi:10.1016/S1473-3099(06)70578-3. PMID 16931408.
- ↑ Brown M, Varia H, Bassett P, Davidson RN, Wall R, Pasvol G (2007). "Prospective study of sputum induction, gastric washing, and bronchoalveolar lavage for the diagnosis of pulmonary tuberculosis in patients who are unable to expectorate". Clin Infect Dis. 44 (11): 1415–20. doi:10.1086/516782. PMID 17479935.
- 1 2 Drobniewski F, Caws M, Gibson A, Young D (2003). "Modern laboratory diagnosis of tuberculosis". Lancet Infect Dis. 3 (3): 141–7. doi:10.1016/S1473-3099(03)00544-9. PMID 12614730.
- ↑ Moore D, Evans C, Gilman R, Caviedes L, Coronel J, Vivar A, Sanchez E, Piñedo Y, Saravia J, Salazar C, Oberhelman R, Hollm-Delgado M, LaChira D, Escombe A, Friedland J (2006). "Microscopic-observation drug-susceptibility assay for the diagnosis of TB". N Engl J Med. 355 (15): 1539–50. doi:10.1056/NEJMoa055524. PMC 1780278
 . PMID 17035648.
. PMID 17035648. - 1 2 Rossi, S. E.; Franquet, T.; Volpacchio, M.; Gimenez, A.; Aguilar, G. (1 May 2005). "Tree-in-Bud Pattern at Thin-Section CT of the Lungs: Radiologic-Pathologic Overview". Radiographics. 25 (3): 789–801. doi:10.1148/rg.253045115. Retrieved 28 May 2012.
- 1 2 Nakamura, R. M.; Einck, L.; Velmonte, M. A.; Kawajiri, K.; Ang, C. F.; Delasllagas, C. E.; Nacy, C. A. (2001-01-01). "Detection of active tuberculosis by an MPB-64 transdermal patch: a field study". Scandinavian Journal of Infectious Diseases. 33 (6): 405–407. doi:10.1080/00365540152029846. ISSN 0036-5548. PMID 11450857.
- ↑ CDC - Guidelines for Using the QuantiFERON-TB Gold Test for Detecting Mycobacterium tuberculosis Infection, United States
- 1 2 3 4 Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, Drobniewski F, Lalvani A (2007). "A systematic review of rapid diagnostic tests for the detection of tuberculosis infection". Health Technol Assess. 11 (3): 1–314. doi:10.3310/hta11030. PMID 17266837.
- ↑ Guerra RL, Hooper NM, Baker JF, et al. (2007). "Use of the Amplified Mycobacterium tuberculosis Direct Test in a Public Health Laboratory: Test Performance and Impact on Clinical Care". Chest. 132 (3): 946–51. doi:10.1378/chest.06-2959. PMID 17573496.
- ↑ "Tibione in the Treatment of Tuberculosis Activity, Dosage and Toxic Manifestations".
- ↑ Ferrara G, et al. (2006). "Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study" (abstract). Lancet. 367 (9519): 1328–1334. doi:10.1016/S0140-6736(06)68579-6. PMID 16631911.
- ↑ Lalvani A (2003). "Spotting latent infection: the path to better tuberculosis control". Thorax. 58 (11): 916–8. doi:10.1136/thorax.58.11.916. PMC 1746498
 . PMID 14586040.
. PMID 14586040. - ↑ Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A (2003). "Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak". Lancet. 361 (9364): 1168–73. doi:10.1016/S0140-6736(03)12950-9. PMID 12686038.
- ↑ Lalvani A, Pathan A, Durkan H, Wilkinson K, Whelan A, Deeks J, Reece W, Latif M, Pasvol G, Hill A (2001). "Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells". Lancet. 357 (9273): 2017–21. doi:10.1016/S0140-6736(00)05115-1. PMID 11438135.
- ↑ "The Science behind T-SPOT.TB Technology".
- ↑ "How T-SPOT.TB Works".
- ↑ Menzies D, Pai M, Comstock G (2007). "Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research". Ann. Intern. Med. 146 (5): 340–54. doi:10.7326/0003-4819-146-5-200703060-00006. PMID 17339619.
- ↑ Kang YA, Lee HW, Hwang SS, et al. (2007). "Usefulness of Whole-Blood Interferon-{gamma} Assay and Interferon-{gamma} Enzyme-Linked Immunospot Assay in the Diagnosis of Active Pulmonary Tuberculosis". Chest. 132 (3): 959–65. doi:10.1378/chest.06-2805. PMID 17505029.
- ↑ Diel R, Loddenkemper R and Nienhaus A. Evidence-Based Comparison of Commercial Interferon-γ Release Assays for Detecting Active TB: A Metaanalysis. Chest; 137 (4):952-968, 2010
- ↑ Gaur RL, Suhosk MM, Banaei N. In vitro immunomodulation of a whole blood IFN-γ release assay enhances T cell responses in subjects with latent tuberculosis infection. PLoS One. 2012
J Infect. 2015 Aug;71(2):276-80. doi: 10.1016/j.jinf.2015.04.004. Environmental temperature impacts on the performance of QuantiFERON-TB Gold In-Tube assays. Jarvis J, Gao Y, de Graaf H, Hughes S, Allan RN, Williams A, Marshall B, Elkington P, Faust SN, Tebruegge M.
Notes
- Medical Examination of Aliens (Refugees and Immigrants) - Division of Global Migration and Quarantine, CDC (website).
- Targeted Tuberculin Testing and Treatment of Latent Tuberculosis Infection 2000 ATS/CDC (fulltext,PDF format) (Updates 2001-2003).
- Lalvani A (November 2003). "Spotting latent infection: the path to better tuberculosis control". Thorax. 58 (11): 916–8. doi:10.1136/thorax.58.11.916. PMC 1746498
 . PMID 14586040.
. PMID 14586040. - Nema V. Tuberculosis diagnostics:Challenges and opportunities. Lung India 2012;29:259-66.
External links
- University of Washington Molecular Diagnosis, Microbiology Division | PCR-based detection in direct tissue samples
- Oxford Immunotec Medical Diagnostics|TB Education and Learning Zone
- Spoligo typing kits (Ocimum Bio Solutions)for detection of TB