DFFA
| DFFA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 |
|||||||||||||||||
| |||||||||||||||||
| Identifiers | |||||||||||||||||
| Aliases | DFFA, DFF-45, DFF1, ICAD, DNA fragmentation factor subunit alpha | ||||||||||||||||
| External IDs | MGI: 1196227 HomoloGene: 3240 GeneCards: DFFA | ||||||||||||||||
| |||||||||||||||||
| RNA expression pattern | |||||||||||||||||
 | |||||||||||||||||
| More reference expression data | |||||||||||||||||
| Orthologs | |||||||||||||||||
| Species | Human | Mouse | |||||||||||||||
| Entrez | |||||||||||||||||
| Ensembl | |||||||||||||||||
| UniProt | |||||||||||||||||
| RefSeq (mRNA) | |||||||||||||||||
| RefSeq (protein) | |||||||||||||||||
| Location (UCSC) | Chr 1: 10.46 – 10.47 Mb | Chr 4: 149.1 – 149.12 Mb | |||||||||||||||
| PubMed search | [1] | [2] | |||||||||||||||
| Wikidata | |||||||||||||||||
| View/Edit Human | View/Edit Mouse |
| DNA Fragmentation factor 45kDa, C terminal domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
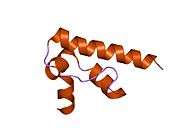 nmr structure of dff-c domain | |||||||||
| Identifiers | |||||||||
| Symbol | DFF-C | ||||||||
| Pfam | PF09033 | ||||||||
| InterPro | IPR015121 | ||||||||
| |||||||||
DNA fragmentation factor subunit alpha (DFFA), also known as Inhibitor of caspase-activated DNase (ICAD), is a protein that in humans is encoded by the DFFA gene.[3][4][5]
Apoptosis is a cell death process that removes toxic and/or useless cells during mammalian development. The apoptotic process is accompanied by shrinkage and fragmentation of the cells and nuclei and degradation of the chromosomal DNA into nucleosomal units. DNA fragmentation factor (DFF) is a heterodimeric protein of 40-kD (DFFB) and 45-kD (DFFA) subunits. DFFA is the substrate for caspase-3 and triggers DNA fragmentation during apoptosis. DFF becomes activated when DFFA is cleaved by caspase-3. The cleaved fragments of DFFA dissociate from DFFB, the active component of DFF. DFFB has been found to trigger both DNA fragmentation and chromatin condensation during apoptosis. Two alternatively spliced transcript variants encoding distinct isoforms have been found for this gene.[5]
The C-terminal domain of DFFA (DFF-C) consists of four alpha-helices, which are folded in a helix-packing arrangement, with alpha-2 and alpha-3 packing against a long C-terminal helix (alpha-4). The main function of this domain is the inhibition of DFFB by binding to its C-terminal catalytic domain through ionic interactions, thereby inhibiting the fragmentation of DNA in the apoptotic process. In addition to blocking the DNase activity of DFFB, the C-terminal region of DFFA is also important for the DFFB-specific folding chaperone activity, as demonstrated by the ability of DFFA to refold DFFB.[6]
Interactions
DFFA has been shown to interact with DFFB.[7][8]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Leek JP, Carr IM, Bell SM, Markham AF, Lench NJ (Jun 1998). "Assignment of the DNA fragmentation factor gene (DFFA) to human chromosome bands 1p36.3→p36.2 by in situ hybridization". Cytogenet Cell Genet. 79 (3–4): 212–3. doi:10.1159/000134725. PMID 9605855.
- ↑ Liu X, Zou H, Slaughter C, Wang X (May 1997). "DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis". Cell. 89 (2): 175–84. doi:10.1016/S0092-8674(00)80197-X. PMID 9108473.
- 1 2 "Entrez Gene: DFFA DNA fragmentation factor, 45kDa, alpha polypeptide".
- ↑ Fukushima K, Kikuchi J, Koshiba S, Kigawa T, Kuroda Y, Yokoyama S (August 2002). "Solution structure of the DFF-C domain of DFF45/ICAD. A structural basis for the regulation of apoptotic DNA fragmentation". J. Mol. Biol. 321 (2): 317–27. doi:10.1016/S0022-2836(02)00588-0. PMID 12144788.
- ↑ Ewing, Rob M; Chu Peter; Elisma Fred; Li Hongyan; Taylor Paul; Climie Shane; McBroom-Cerajewski Linda; Robinson Mark D; O'Connor Liam; Li Michael; Taylor Rod; Dharsee Moyez; Ho Yuen; Heilbut Adrian; Moore Lynda; Zhang Shudong; Ornatsky Olga; Bukhman Yury V; Ethier Martin; Sheng Yinglun; Vasilescu Julian; Abu-Farha Mohamed; Lambert Jean-Philippe; Duewel Henry S; Stewart Ian I; Kuehl Bonnie; Hogue Kelly; Colwill Karen; Gladwish Katharine; Muskat Brenda; Kinach Robert; Adams Sally-Lin; Moran Michael F; Morin Gregg B; Topaloglou Thodoros; Figeys Daniel (2007). "Large-scale mapping of human protein–protein interactions by mass spectrometry". Mol. Syst. Biol. England. 3 (1): 89. doi:10.1038/msb4100134. PMC 1847948
 . PMID 17353931.
. PMID 17353931. - ↑ McCarty, J S; Toh S Y; Li P (Oct 1999). "Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity". Biochem. Biophys. Res. Commun. UNITED STATES. 264 (1): 176–80. doi:10.1006/bbrc.1999.1497. ISSN 0006-291X. PMID 10527860.
Further reading
- Nakanuma Y, Tsuneyama K, Sasaki M, Harada K (2000). "Destruction of bile ducts in primary biliary cirrhosis". Baillière's best practice & research. Clinical gastroenterology. 14 (4): 549–70. doi:10.1053/bega.2000.0103. PMID 10976014.
- Maruyama K, Sugano S (1994). "Oligo-capping: a simple method to replace the cap structure of eukaryotic mRNAs with oligoribonucleotides". Gene. 138 (1–2): 171–4. doi:10.1016/0378-1119(94)90802-8. PMID 8125298.
- Suzuki Y, Yoshitomo-Nakagawa K, Maruyama K, et al. (1997). "Construction and characterization of a full length-enriched and a 5'-end-enriched cDNA library". Gene. 200 (1–2): 149–56. doi:10.1016/S0378-1119(97)00411-3. PMID 9373149.
- Enari M, Sakahira H, Yokoyama H, et al. (1998). "A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD". Nature. 391 (6662): 43–50. doi:10.1038/34112. PMID 9422506.
- Liu X, Zou H, Widlak P, et al. (1999). "Activation of the apoptotic endonuclease DFF40 (caspase-activated DNase or nuclease). Oligomerization and direct interaction with histone H1". J. Biol. Chem. 274 (20): 13836–40. doi:10.1074/jbc.274.20.13836. PMID 10318789.
- Gu J, Dong RP, Zhang C, et al. (1999). "Functional interaction of DFF35 and DFF45 with caspase-activated DNA fragmentation nuclease DFF40". J. Biol. Chem. 274 (30): 20759–62. doi:10.1074/jbc.274.30.20759. PMID 10409614.
- Oh JJ, Grosshans DR, Wong SG, Slamon DJ (1999). "Identification of differentially expressed genes associated with HER-2/neu overexpression in human breast cancer cells". Nucleic Acids Res. 27 (20): 4008–17. doi:10.1093/nar/27.20.4008. PMC 148668
 . PMID 10497265.
. PMID 10497265. - McCarty JS, Toh SY, Li P (1999). "Study of DFF45 in its role of chaperone and inhibitor: two independent inhibitory domains of DFF40 nuclease activity". Biochem. Biophys. Res. Commun. 264 (1): 176–80. doi:10.1006/bbrc.1999.1497. PMID 10527860.
- McCarty JS, Toh SY, Li P (1999). "Multiple domains of DFF45 bind synergistically to DFF40: roles of caspase cleavage and sequestration of activator domain of DFF40". Biochem. Biophys. Res. Commun. 264 (1): 181–5. doi:10.1006/bbrc.1999.1498. PMID 10527861.
- Lugovskoy AA, Zhou P, Chou JJ, et al. (2000). "Solution structure of the CIDE-N domain of CIDE-B and a model for CIDE-N/CIDE-N interactions in the DNA fragmentation pathway of apoptosis". Cell. 99 (7): 747–55. doi:10.1016/S0092-8674(00)81672-4. PMID 10619428.
- Otomo T, Sakahira H, Uegaki K, et al. (2000). "Structure of the heterodimeric complex between CAD domains of CAD and ICAD". Nat. Struct. Biol. 7 (8): 658–62. doi:10.1038/77957. PMID 10932250.
- Xerri L, Palmerini F, Devilard E, et al. (2000). "Frequent nuclear localization of ICAD and cytoplasmic co-expression of caspase-8 and caspase-3 in human lymphomas". J. Pathol. 192 (2): 194–202. doi:10.1002/1096-9896(2000)9999:9999<::AID-PATH685>3.0.CO;2-M. PMID 11004695.
- Zhou P, Lugovskoy AA, McCarty JS, et al. (2001). "Solution structure of DFF40 and DFF45 N-terminal domain complex and mutual chaperone activity of DFF40 and DFF45". Proc. Natl. Acad. Sci. U.S.A. 98 (11): 6051–5. doi:10.1073/pnas.111145098. PMC 33420
 . PMID 11371636.
. PMID 11371636. - Sharif-Askari E, Alam A, Rhéaume E, et al. (2001). "Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation". EMBO J. 20 (12): 3101–13. doi:10.1093/emboj/20.12.3101. PMC 150191
 . PMID 11406587.
. PMID 11406587. - Tsukada T, Watanabe M, Yamashima T (2002). "Implications of CAD and DNase II in ischemic neuronal necrosis specific for the primate hippocampus". J. Neurochem. 79 (6): 1196–206. doi:10.1046/j.1471-4159.2001.00679.x. PMID 11752060.
- Abel F, Sjöberg RM, Ejeskär K, et al. (2002). "Analyses of apoptotic regulators CASP9 and DFFA at 1P36.2, reveal rare allele variants in human neuroblastoma tumours". Br. J. Cancer. 86 (4): 596–604. doi:10.1038/sj.bjc.6600111. PMC 2375272
 . PMID 11870543.
. PMID 11870543. - Charrier L, Jarry A, Toquet C, et al. (2002). "Growth phase-dependent expression of ICAD-L/DFF45 modulates the pattern of apoptosis in human colonic cancer cells". Cancer Res. 62 (7): 2169–74. PMID 11929840.
This article incorporates text from the public domain Pfam and InterPro IPR015121
