Inclusion compound
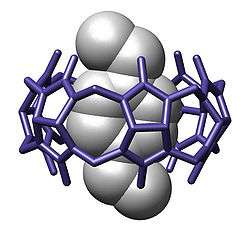
In host-guest chemistry, an inclusion compound is a complex in which one chemical compound (the "host") forms a cavity in which molecules of a second "guest" compound are located. The definition of inclusion compounds is very broad, extending to channels formed between molecules in a crystal lattice in which guest molecules can fit. If the spaces in the host lattice are enclosed on all sides so that the guest species is ‘trapped’ as in a cage, the compound is known as a clathrate. In molecular encapsulation, a guest molecule is actually trapped inside another molecule.
Cyclodextrin inclusion compounds
Inclusion complexes are formed between cyclodextrins and different guest molecules such as ferrocene. When a solution of both compounds in a 2:1 ratio in water is under hydrothermal conditions at 100 degree C for 2 days and then allowed to rest for 10 hours at room temperature orange-yellow crystals form. X-ray diffraction analysis of these crystals reveals a 4:5 inclusion complex with 4 molecules of ferrocene included in the cavity of 4 cyclodextrine molecules and with the fifth ferrocene molecule sandwiched between two stacks of ferrocene - cyclodextrine dimers.
Cyclodextrin also forms inclusion compounds with fragrance molecules. As a result, the fragrance molecules have a reduced vapor pressure and are more stable towards exposure to light and air. When incorporated into textiles the fragrance lasts much longer due to the slow-release action.
External links
- IUPAC Gold Book definition
References
- ^ alpha-Cyclodextrin dimer complexes of dopamine and levodopa derivatives to assess drug delivery to the central nervous system: ADME and molecular docking studies S. Shityakov, J. Broscheit and C. Förster International Journal of Nanomedicine, 2012, (7), 3211 - 3219 Abstract
- ^ Pharmacokinetic delivery and metabolizing rate of nicardipine incorporated in hydrophilic and hydrophobic cyclodextrins using two-compartment mathematical model S. Shityakov and C. Förster ScientificWorldJournal, 2013, 131358 Abstract
- ^ Ionization states, cellular toxicity and molecular modeling studies of midazolam complexed with trimethyl-beta-cyclodextrin S. Shityakov, T. Sohajda, I. Puskás, N. Roewer, C. Förster C and J. A. Broscheit Molecules, 2014, (10), 16861 - 16876 Abstract
- ^ A unique tetramer of 4:5 -cyclodextrin–ferrocene in the solid state Yu Liu, Rui-Qin Zhong, Heng-Yi Zhang and Hai-Bin Song Chemical Communications, 2005, (17), 2211 - 2213 Abstract
- ^ Fragrance-release Property of β-Cyclodextrin Inclusion Compounds and their Application in Aromatherapy C. X. Wang, Sh. L. Chen Journal of Industrial Textiles, Vol. 34, No. 3, 157-166 (2005) Abstract